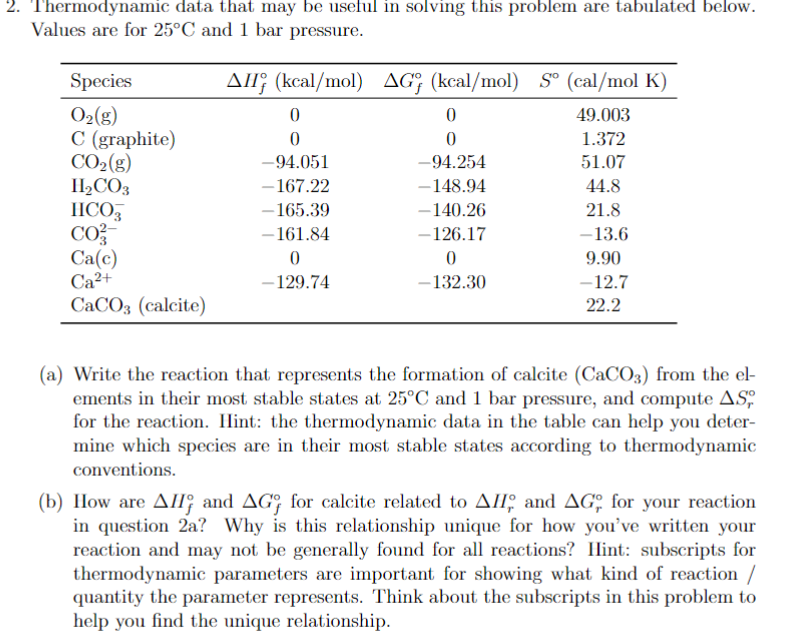
Question: Thermodynamic data that may be useful in solving this problem are tabulated below. Values are for 2 5 C and 1 bar pressure. ( a
Thermodynamic data that may be useful in solving this problem are tabulated below.
Values are for and bar pressure.
a Write the reaction that represents the formation of calcite from the el
ements in their most stable states at and bar pressure, and compute
for the reaction. Ilint: the thermodynamic data in the table can help you deter
mine which species are in their most stable states according to thermodynamic
conventions.
b How are and for calcite related to and for your reaction
in question a Why is this relationship unique for how you've written your
reaction and may not be generally found for all reactions? Ilint: subscripts for
thermodynamic parameters are important for showing what kind of reaction
quantity the parameter represents. Think about the subscripts in this problem to
help you find the unique relationship.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


