Question: please help with steps for all parts. will upvote for sure! 1. Assuming the validity of Raoult's Law and the shortcut vapour pressure equation, do
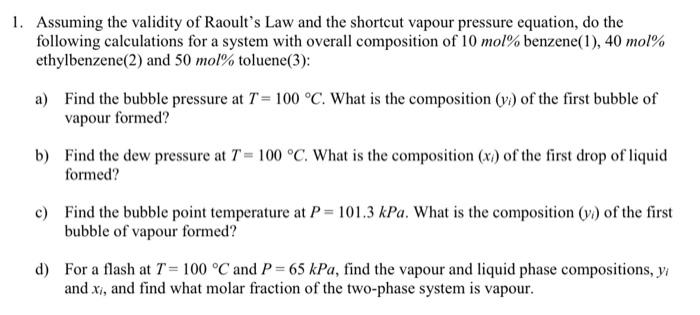
1. Assuming the validity of Raoult's Law and the shortcut vapour pressure equation, do the following calculations for a system with overall composition of 10mol% benzene(1), 40mol% ethylbenzene(2) and 50mol% toluene(3): a) Find the bubble pressure at T=100C. What is the composition (yi) of the first bubble of vapour formed? b) Find the dew pressure at T=100C. What is the composition (xi) of the first drop of liquid formed? c) Find the bubble point temperature at P=101.3kPa. What is the composition (yi) of the first bubble of vapour formed? d) For a flash at T=100C and P=65kPa, find the vapour and liquid phase compositions, yi and xi, and find what molar fraction of the two-phase system is vapour
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


