Question: please help with table 5 !! hot water was added to cold water. Table 5 is at the last picture thank you !! Heat Capacity
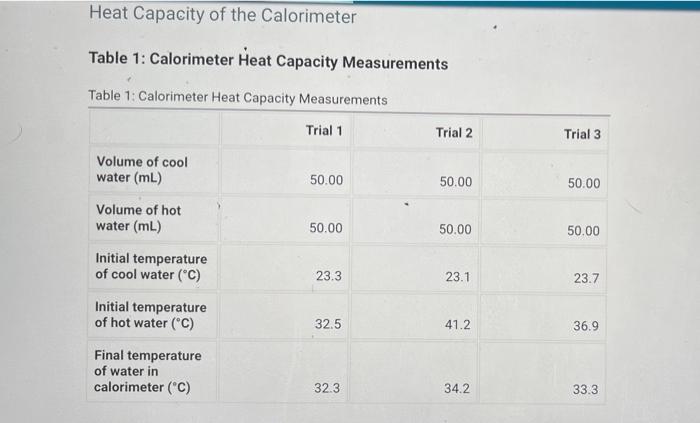
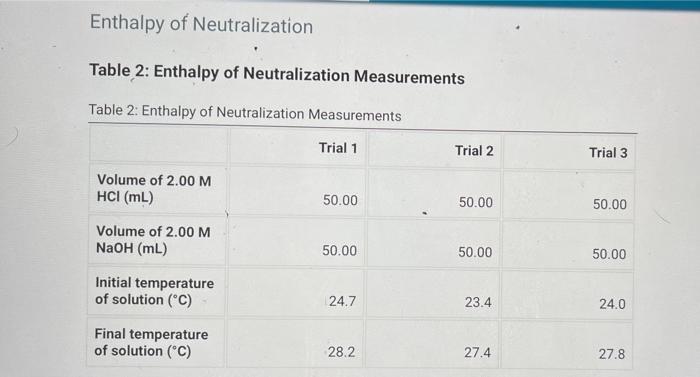
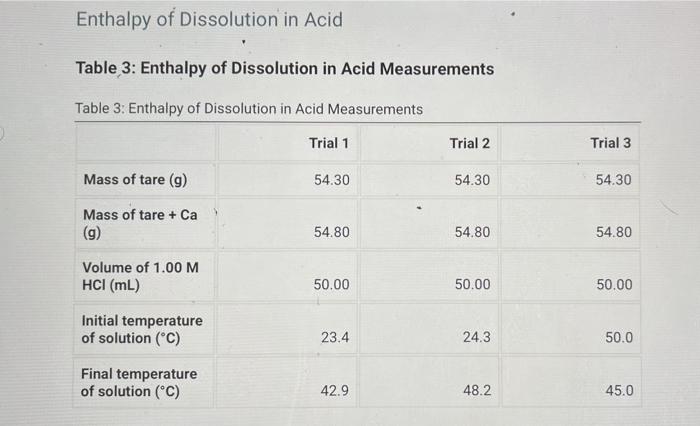
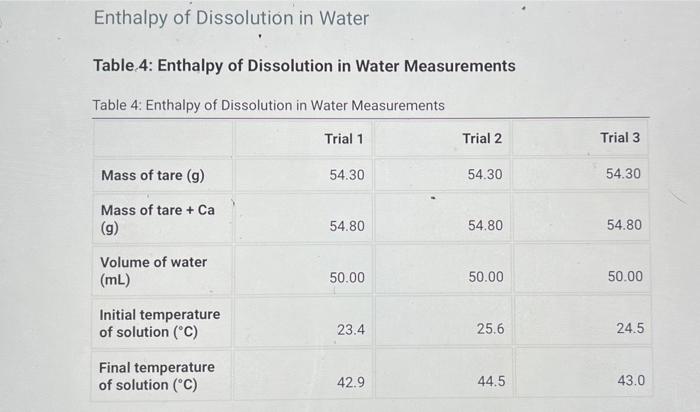
Heat Capacity of the Calorimeter Table 1: Calorimeter Heat Capacity Measurements Table 1: Calorimeter Heat Capacity Measurements Trial 1 Trial 2 Trial 3 Volume of cool water (mL) 50.00 50.00 50.00 Volume of hot water (ml) 50.00 50.00 50.00 23.3 23.1 23.7 Initial temperature of cool water (C) Initial temperature of hot water (C) 32.5 41.2 36.9 Final temperature of water in calorimeter (C) 32.3 34.2 33.3 Enthalpy of Neutralization Table 2: Enthalpy of Neutralization Measurements Table 2: Enthalpy of Neutralization Measurements Trial 1 Trial 2 Trial 3 Volume of 2.00 M HCI (mL) 50.00 50.00 50.00 Volume of 2.00 M NaOH (mL) 50.00 50.00 50.00 Initial temperature of solution (C) 24.7 23.4 24.0 Final temperature of solution (C) 28.2 27.4 27.8 Enthalpy of Dissolution in Acid Table 3: Enthalpy of Dissolution in Acid Measurements Table 3: Enthalpy of Dissolution in Acid Measurements Trial 1 Trial 2 Trial 3 Mass of tare (9) 54.30 54.30 54.30 Mass of tare + Ca (9) 54.80 54.80 54.80 Volume of 1.00 M HCI (mL) 50.00 50.00 50.00 Initial temperature of solution (C) 23.4 24.3 50.0 Final temperature of solution (C) 42.9 48.2 45.0 Enthalpy of Dissolution in Water Table 4: Enthalpy of Dissolution in Water Measurements Table 4: Enthalpy of Dissolution in Water Measurements Trial 1 Trial 2 Trial 3 Mass of tare (9) 54.30 54.30 54.30 Mass of tare + Ca (9) 54.80 54.80 54.80 Volume of water (ml) 50.00 50.00 50.00 Initial temperature of solution (C) 23.4 25.6 24.5 Final temperature of solution (C) 42.9 44.5 43.0 Table 5: Calculated results Table view List view Table 5Calculated results Trial 1 Trial 2 Trial 3 Average Heat capacity of calorimeter (J/C) Enthalpy of neutralization (kJ/mol) Enthalpy of dissolution of calcium in acid (kJ/mol) Enthalpy of dissolution of calcium in water (kJ/mol)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


