Question: Please help with the next question as well i will rate! The following thermochemical equation is for the reaction of hydrogen sulfide(g) with oxygen(g) to
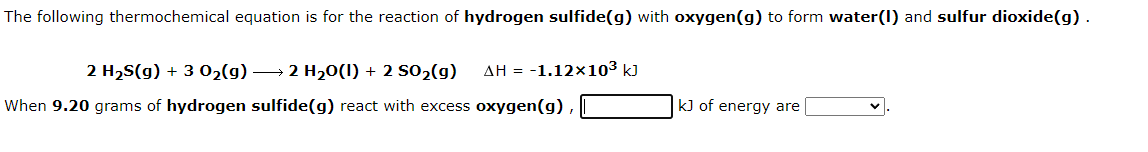 Please help with the next question as well i will rate!
Please help with the next question as well i will rate!
The following thermochemical equation is for the reaction of hydrogen sulfide(g) with oxygen(g) to form water(I) and sulfur dioxide(g). 2 H2(g) + 302(g) 2 H20(1) + 2 502(g) AH = -1.12x103 kJ When 9.20 grams of hydrogen sulfide(g) react with excess oxygen(g), k) of energy are In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a metal. She heats 19.9 grams of gold to 98.03C and then drops it into a cup containing 79.9 grams of water at 21.51. She measures the final temperature to be 22.12C. Assuming that all of the heat is transferred to the water, she calculates the specific heat of gold to be J/gC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


