Question: Please help with these 3 questions! Thank you! Incorrect Question 6 0/0.75 pts What is the result of the following calculation to the correct number
Please help with these 3 questions! Thank you!
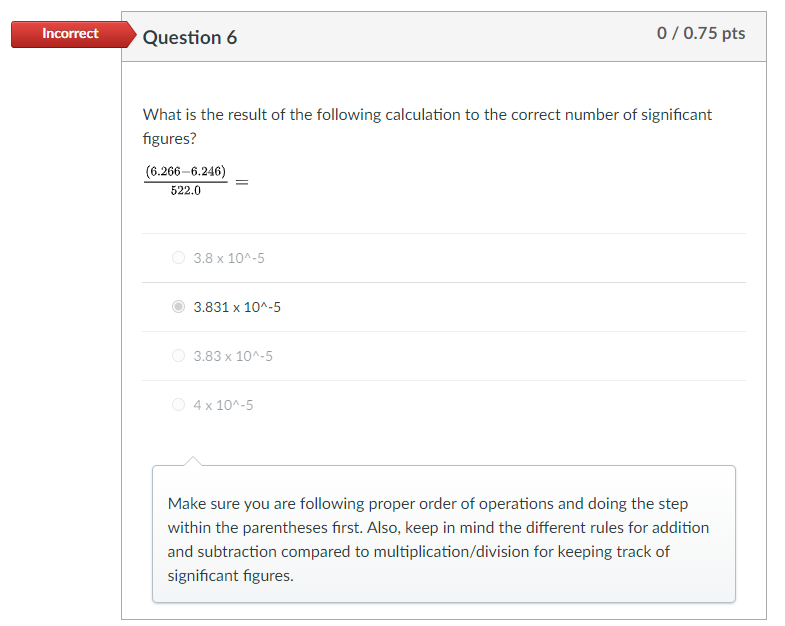
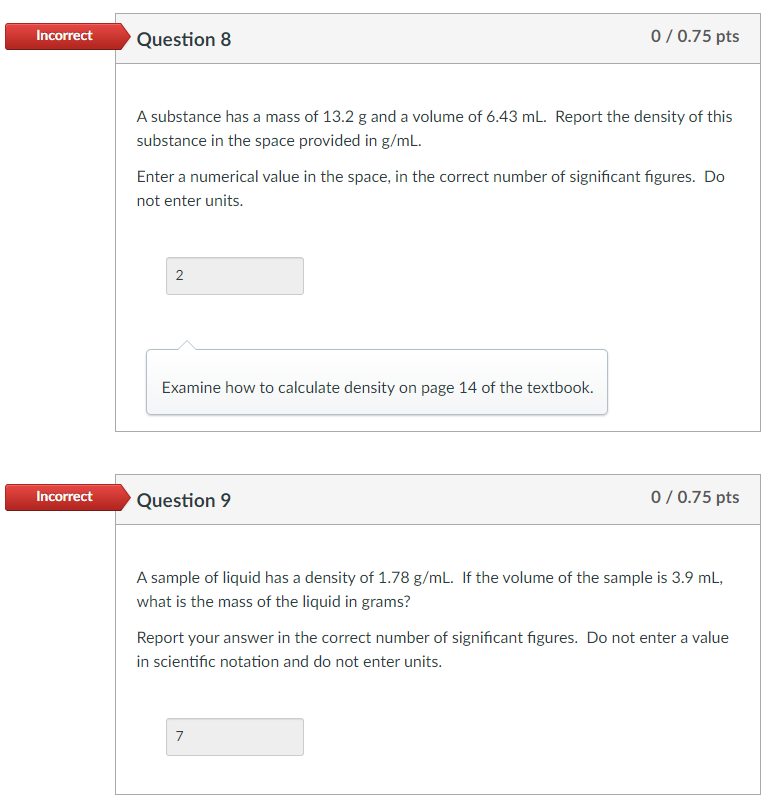
Incorrect Question 6 0/0.75 pts What is the result of the following calculation to the correct number of significant figures? (6.266-6.246) 522.0 3.8 x 10^-5 3.831 x 10^-5 3.83 x 10^-5 4 x 10^-5 Make sure you are following proper order of operations and doing the step within the parentheses first. Also, keep in mind the different rules for addition and subtraction compared to multiplication/division for keeping track of significant figures. Incorrect Question 8 0/0.75 pts A substance has a mass of 13.2 g and a volume of 6.43 ml. Report the density of this substance in the space provided in g/mL. Enter a numerical value in the space, in the correct number of significant figures. Do not enter units. 2 Examine how to calculate density on page 14 of the textbook. Incorrect Question 9 0/0.75 pts A sample of liquid has a density of 1.78 g/ml. If the volume of the sample is 3.9 ml, what is the mass of the liquid in grams? Report your answer in the correct number of significant figures. Do not enter a value in scientific notation and do not enter units. 7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


