Question: please help with these! (even explaining the concepts is fine!!) 1. Analyze the two sets of Newman projections and determine the relationship between each set.
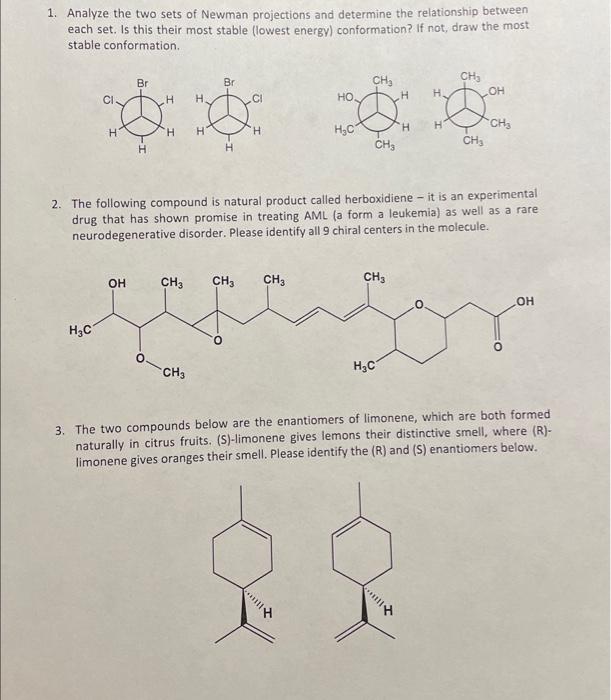
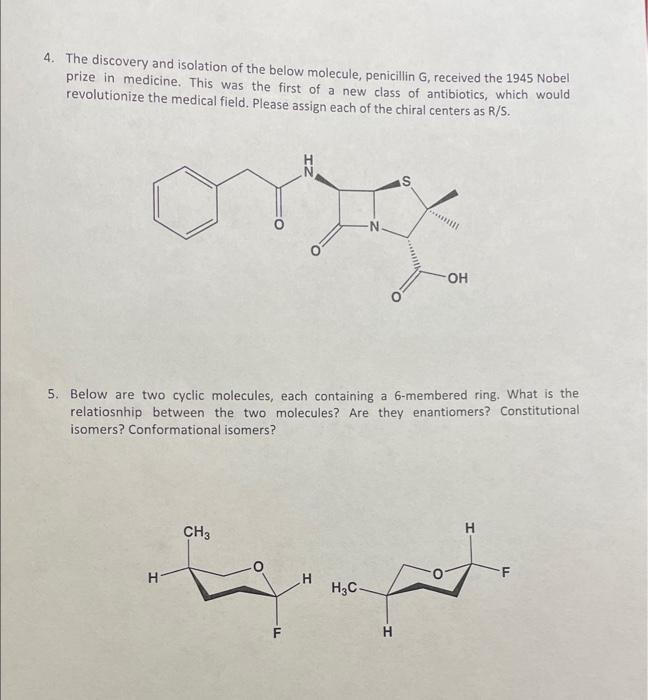
1. Analyze the two sets of Newman projections and determine the relationship between each set. Is this their most stable (lowest energy) conformation? If not, draw the most stable conformation. 2. The following compound is natural product called herboxidiene - it is an experimental drug that has shown promise in treating AML (a form a leukemia) as well as a rare neurodegenerative disorder. Please identify all 9 chiral centers in the molecule. 3. The two compounds below are the enantiomers of limonene, which are both formed naturally in citrus fruits. (S)-limonene gives lemons their distinctive smell, where (R)limonene gives oranges their smell. Please identify the (R) and (S) enantiomers below. 4. The discovery and isolation of the below molecule, penicillin G, received the 1945 Nobel prize in medicine. This was the first of a new class of antibiotics, which would revolutionize the medical field. Please assign each of the chiral centers as R/S. 5. Below are two cyclic molecules, each containing a 6-membered ring. What is the relatiosnhip between the two molecules? Are they enantiomers? Constitutional isomers? Conformational isomers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


