Question: please help with these questions using this data Post Lab Answer the following questions on a separate sheet and attach to your completed lab. 1.
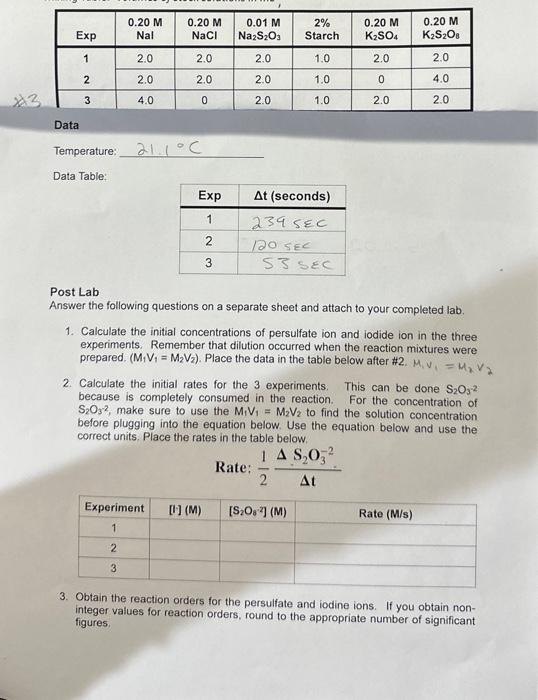

Post Lab Answer the following questions on a separate sheet and attach to your completed lab. 1. Calculate the initial concentrations of persulfate ion and iodide ion in the three experiments. Remember that dilution occurred when the reaction mixtures were prepared. (M1V1=M2V2). Place the data in the table below after #2. M1v1=M2V2 2. Calculate the initial rates for the 3 experiments. This can be done S2O32 because is completely consumed in the reaction. For the concentration of S2O32, make sure to use the M1V1=M2V2 to find the solution concentration before plugging into the equation below. Use the equation below and use the correct units. Place the rates in the table below. Rate: 21tS2O32 3. Obtain the reaction orders for the persulfate and iodine ions. If you obtain noninteger values for reaction orders, round to the appropriate number of significant figures. 4. What is the correct rate law? 5. Calculate the rate constant k in the three experiments and obtain the average and standard deviation. Give correct units. 6. Discuss your results. Do you think your results were good? What could you have done to improve your results
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


