Question: please !!! help with these two i cannot figure out thr correct answer !!! Use the integrated rate law to calculate time elapsed. SO2Cl2(g)SO2(g)+Cl2(g) How
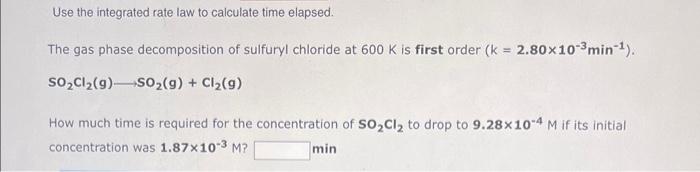

Use the integrated rate law to calculate time elapsed. SO2Cl2(g)SO2(g)+Cl2(g) How much time is required for the concentration of SO2Cl2 to drop to 9.28104M if its initial concentration was 1.87103M ? min Use the integrated rate law to determine the rate constant. The decomposition of nitrous oxide at 565C is second order in N2O. N2O(g)N2(g)+1/2O2(g) During one experiment it was found that the N2O concentration dropped from 1.68M at the beginning of the experiment to 0.739M in 616.2 seconds. What is the value of the rate constant for the reaction at this temperature? M1s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


