Question: please help with these two questions! 4.60 Knowing what you do about electronegativity, the polarity of covalent bonds, and hydrogen bonding, would you expect an
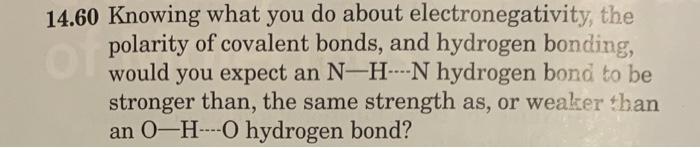
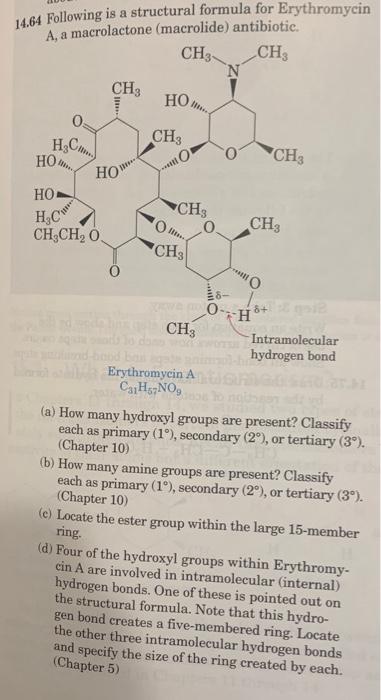
4.60 Knowing what you do about electronegativity, the polarity of covalent bonds, and hydrogen bonding, would you expect an NHN hydrogen bond to be stronger than, the same strength as, or weaker than an OHO hydrogen bond? 14.64 Following is a structural formula for Erythromycin 4 a macrolactone (macrolide) antibiotic. C31H57NO9 (a) How many hydroxyl groups are present? Classify each as primary (1), secondary (2), or tertiary (3). (Chapter 10) (b) How many amine groups are present? Classify each as primary (1), secondary (2), or tertiary (3). (Chapter 10) (c) Locate the ester group within the large 15-member ring. (d) Four of the hydroxyl groups within Erythromycin A are involved in intramolecular (internal) hydrogen bonds. One of these is pointed out on the structural formula. Note that this hydrogen bond creates a five-membered ring. Locate the other three intramolecular hydrogen bonds and specify the size of the ring created by each. (Chapter 5)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


