Question: Please help with this! Clear and step by step please with explain. Biochemistry. A new amino acid has been isolated that has been titrated to
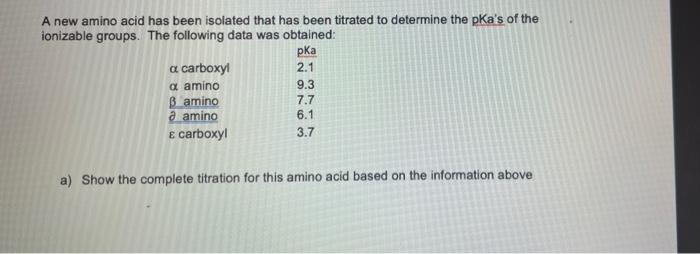
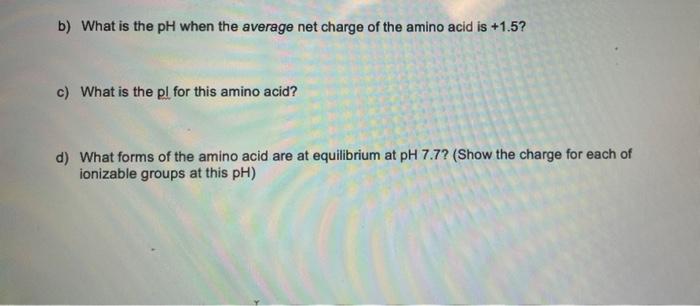
A new amino acid has been isolated that has been titrated to determine the pka's of the ionizable groups. The following data was obtained: pka a carboxyi 2.1 a amino 9.3 B amino 7.7 a amino 6.1 E carboxyl 3.7 a) Show the complete titration for this amino acid based on the information above b) What is the pH when the average net charge of the amino acid is +1.5? c) What is the pl for this amino acid? d) What forms of the amino acid are at equilibrium at pH 7.7? (Show the charge for each of ionizable groups at this pH)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


