Question: Please help with this question. Please notice the section about the addition of 1.000 atm of argon into the system. Thank you. Consider the partial
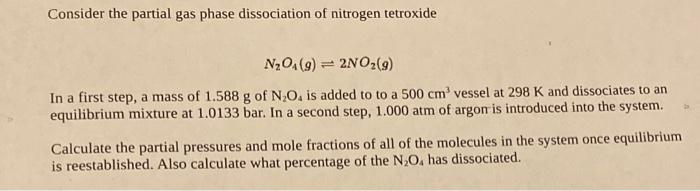
Consider the partial gas phase dissociation of nitrogen tetroxide N2O4(g)2NO2(g) In a first step, a mass of 1.588g of N2O4 is added to to a 500cm3 vessel at 298K and dissociates to an equilibrium mixture at 1.0133 bar. In a second step, 1.000atm of argon is introduced into the system. Calculate the partial pressures and mole fractions of all of the molecules in the system once equilibrium is reestablished. Also calculate what percentage of the N2O4 has dissociated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


