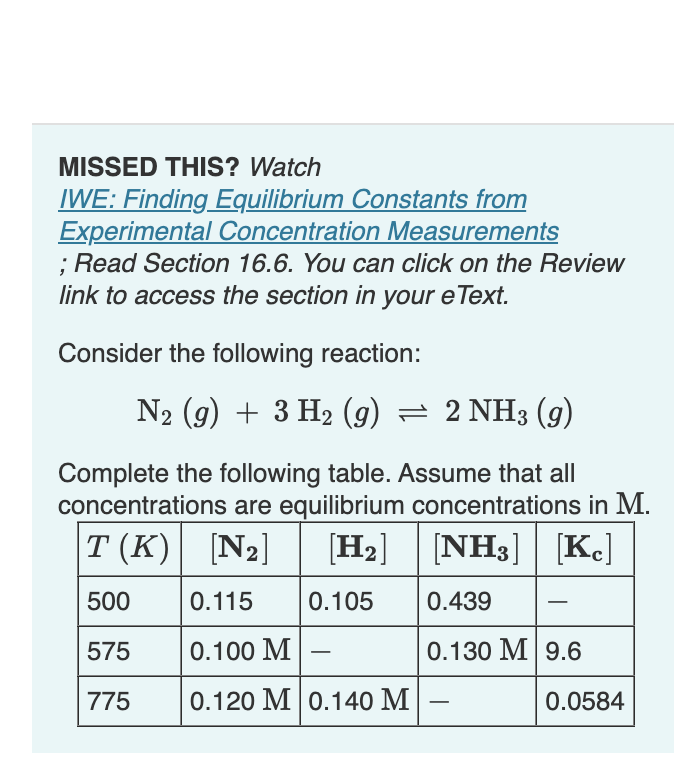
Question: please helpp MISSED THIS? Watch IWE: Finding Equilibrium Constants from Experimental Concentration Measurements ; Read Section 16.6. You can click on the Review link to
 please helpp
please helpp
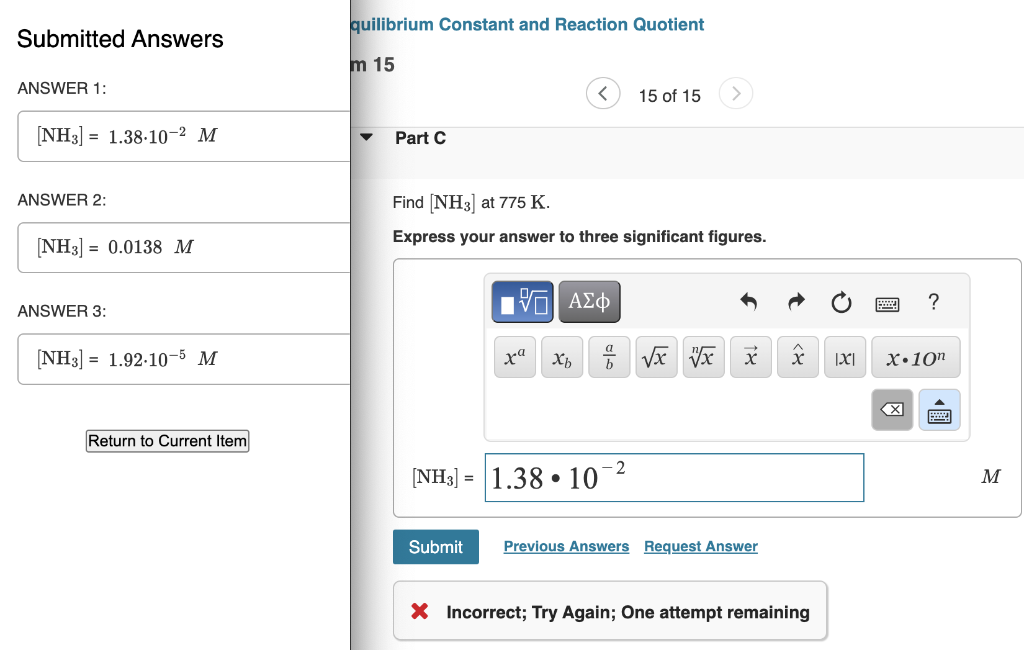
MISSED THIS? Watch IWE: Finding Equilibrium Constants from Experimental Concentration Measurements ; Read Section 16.6. You can click on the Review link to access the section in your eText. Consider the following reaction: N2(g)+3H2(g)2NH3(g) Complete the following table. Assume that all concentrations are equilibrium concentrations in M. [NH3]=1.38102MPartC ANSWER 2: Find [NH3] at 775K. [NH3]=0.0138M Express your answer to three significant figures. ANSWER 3 : [NH3]=1.92105M [r X Incorrect; Try Again; One attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


