Question: please i need an answer thank you 3. Using the table below and answer the following questions: (1) Calculate the diffusion coefficient for Cu in
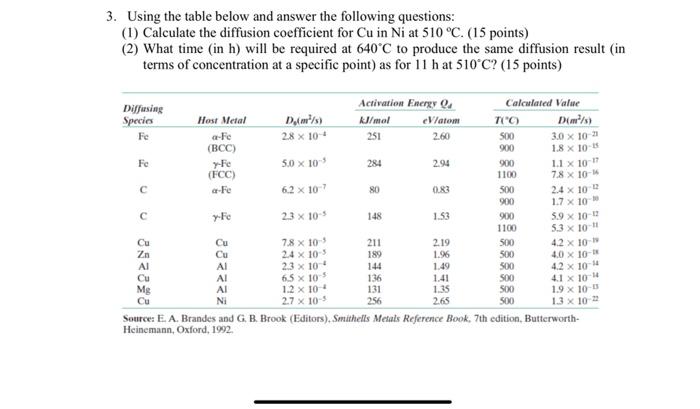
3. Using the table below and answer the following questions: (1) Calculate the diffusion coefficient for Cu in Ni at 510 C. (15 points) (2) What time in h) will be required at 640C to produce the same diffusion result (in terms of concentration at a specific point) as for 11 h at 510C? (15 points) Diffusing Activation Energy Calculated Value Species Host Metal Dykn/s) kJ/mol V/atom TIC D(m/) Fe a-Fe 28 x 10 251 2.60 500 (BCC) 3.0 x 10-21 900 1.8 X 10-15 Fe Fe 5.0 x 10 284 2.94 900 (FCC) 11 X 10-11 1100 7.8 X 10- Fe 6.2 x 10- 80 0.83 500 24 x 10-6 900 1.7 x 10 23 x 10-5 148 1.53 900 5.9 x 10- 1100 53 x 10" Cu 7.8 x 10 2.19 500 Zn Cu 2.4 x 10-5 4.2 x 10 189 1.96 Al 500 AI 23 x 10- 4.0 x 10- 144 1.49 500 AI 6.5 x 10-5 4.2 x 10-4 136 1.41 500 Me AI 1.2 x 10+ 41 X 10-14 131 1.35 500 Cu NI 1.9 x 10-6 2.7 x 10-5 256 2.65 500 13 x 10-22 Source: E. A. Brandes and G. B. Brook (Editors), Smithells Metals Reference Book, 7th edition, Butterworth- Heinemann, Oxford, 1992 re 211 852828
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


