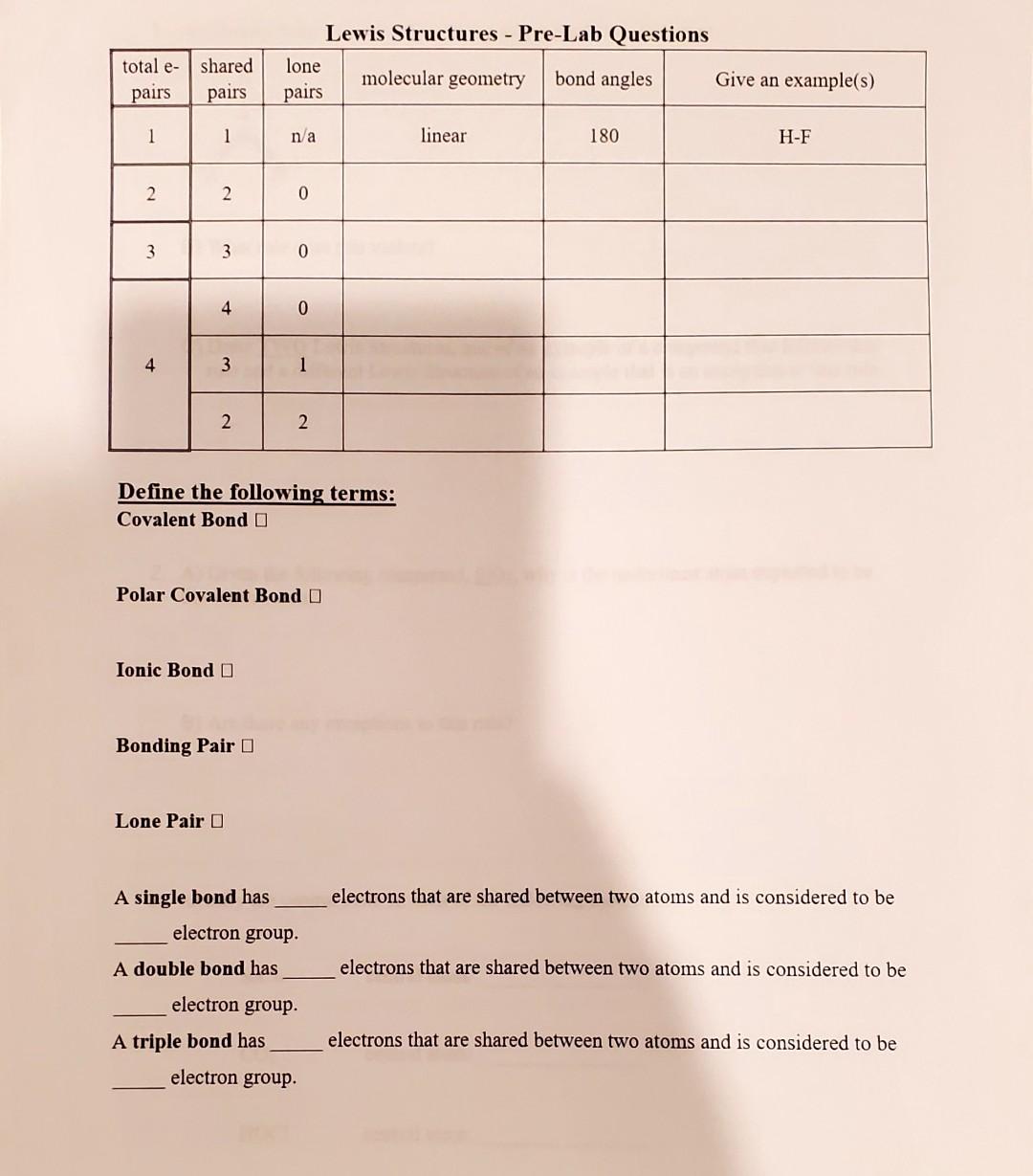
Question: please I need help with the answers Lewis Structures - Pre-Lab Ouestions Define the following terms: Covalent Bond Polar Covalent Bond Ionic Bond Bonding Pair

please I need help with the answers
Lewis Structures - Pre-Lab Ouestions Define the following terms: Covalent Bond Polar Covalent Bond Ionic Bond Bonding Pair Lone Pair A single bond has electrons that are shared between two atoms and is considered to be electron group. A double bond has electrons that are shared between two atoms and is considered to be electron group. A triple bond has electrons that are shared between two atoms and is considered to be electron group. 1. A) Identify what is incorrect about the following Lewis Structure. B) What rule does this violate? C) Draw TWO Lewis Structures, one of an example of a compound that follows this rule and a different Lewis Structure of an example that is an exception to this rule. 2. A) Given the following compound, SiO2, why is the underlined atom expected to be the central atom? B) Are there any exceptions to this rule? 3. Identify the central atom in each of the following molecules: SiCl4 central atom: CO2 central atom: HOCl central atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


