Question: please I need the answer quickly (5 marks) The following temperature composition data were obtained for a mixture of two liquids A and B at
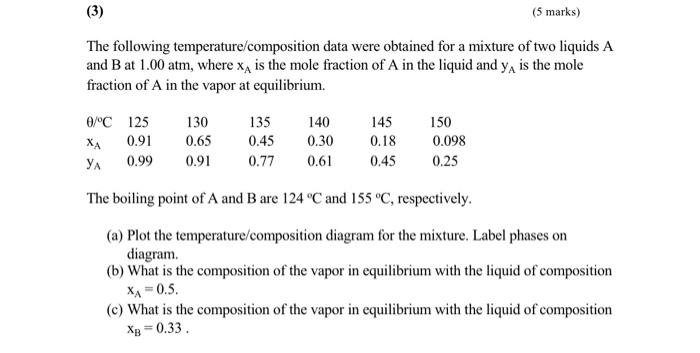
(5 marks) The following temperature composition data were obtained for a mixture of two liquids A and B at 1.00 atm, where xa is the mole fraction of A in the liquid and ya is the mole fraction of A in the vapor at equilibrium. 0/C 125 XA 0.91 YA 0.99 130 0.65 0.91 135 0.45 0.77 140 0.30 0.61 145 0.18 0.45 150 0.098 0.25 The boiling point of A and B are 124 C and 155 C, respectively. (a) Plot the temperature/composition diagram for the mixture. Label phases on diagram. (b) What is the composition of the vapor in equilibrium with the liquid of composition XA = 0.5. (e) What is the composition of the vapor in equilibrium with the liquid of composition XB = 0.33
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


