Question: Please I want the answer in details without using exce (3) For the acetone (1)/methanol (2) system a vapor mixture for which Z1=0.25 and Z2=0.75
Please I want the answer in details without using exce
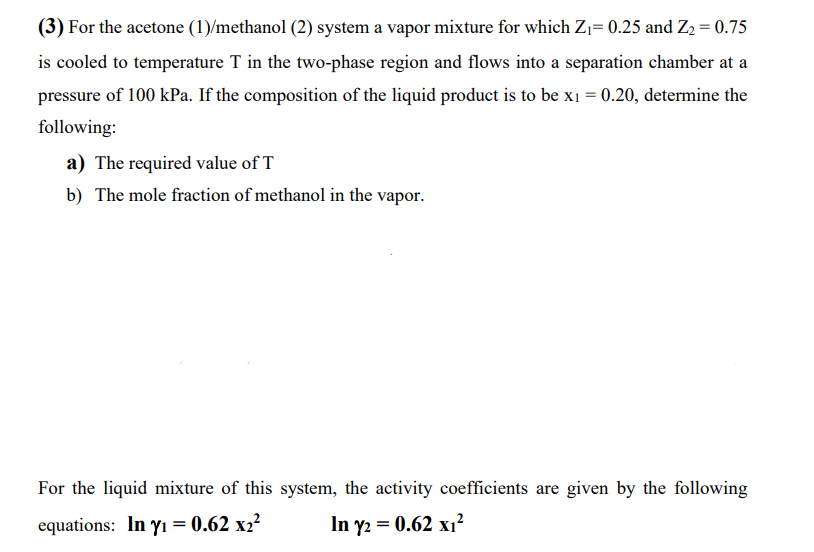
(3) For the acetone (1)/methanol (2) system a vapor mixture for which Z1=0.25 and Z2=0.75 is cooled to temperature T in the two-phase region and flows into a separation chamber at a pressure of 100kPa. If the composition of the liquid product is to be x1=0.20, determine the following: a) The required value of T b) The mole fraction of methanol in the vapor. For the liquid mixture of this system, the activity coefficients are given by the following equations: ln1=0.62x22ln2=0.62x2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


