Question: Please identify the MAJOR contributing resonance structure (most significant) in this case: D Please identify ALL MINOR contributing resonance structures (least significant) in this case:
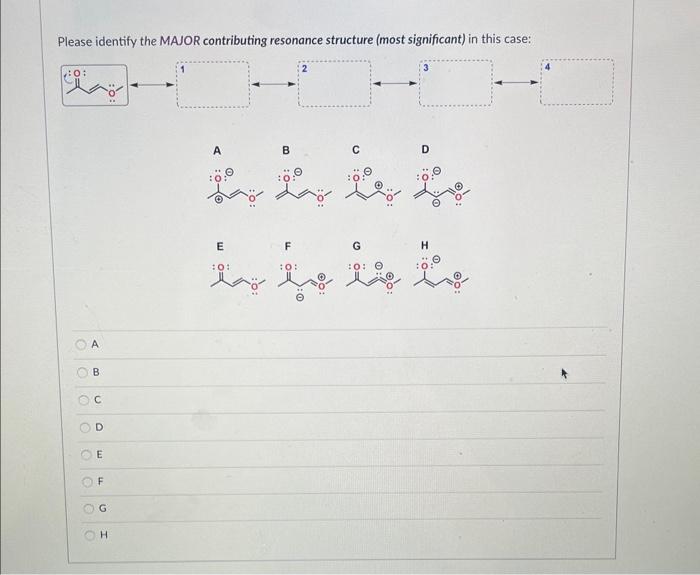
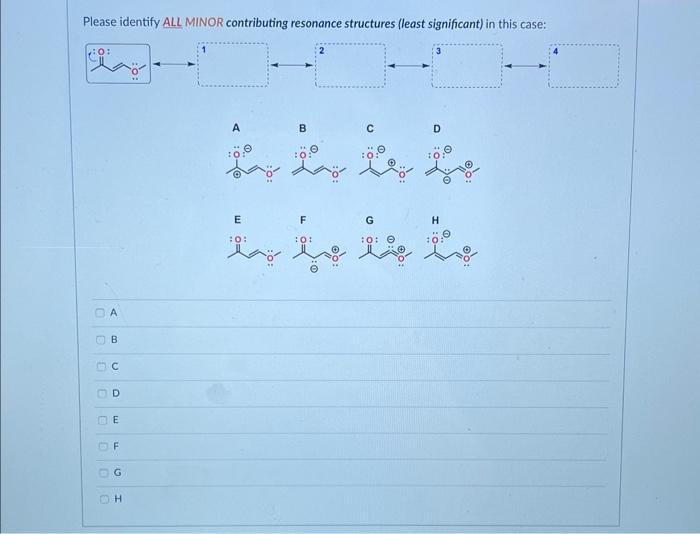
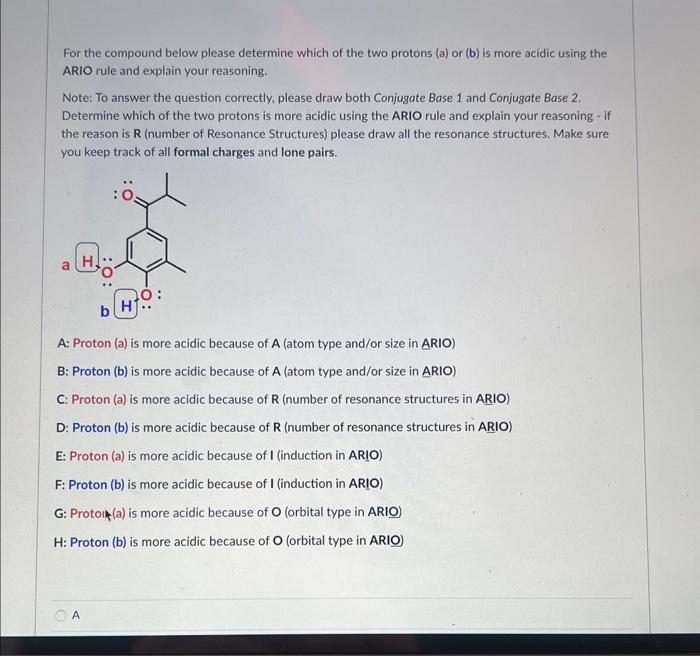
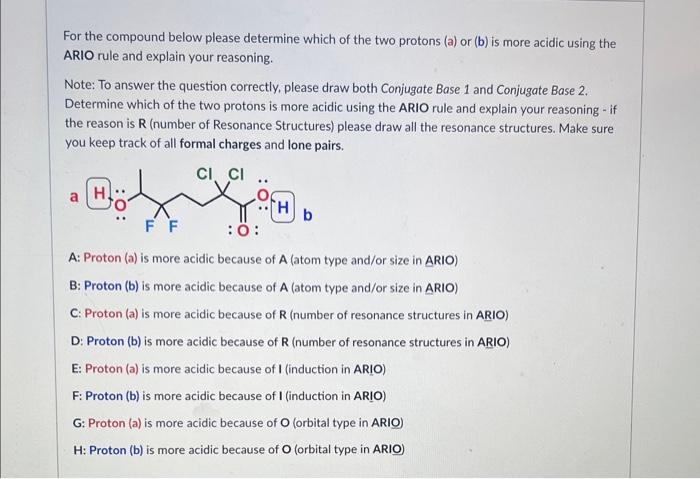
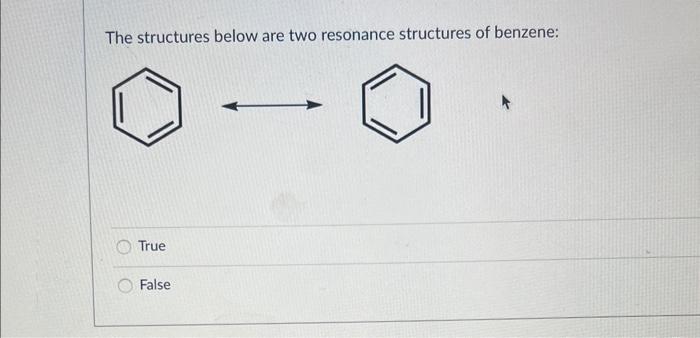
Please identify the MAJOR contributing resonance structure (most significant) in this case: D Please identify ALL MINOR contributing resonance structures (least significant) in this case: For the compound below please determine which of the two protons (a) or (b) is more acidic using the ARIO rule and explain your reasoning. Note: To answer the question correctly, please draw both Conjugate Base 1 and Conjugate Base 2. Determine which of the two protons is more acidic using the ARIO rule and explain your reasoning - if the reason is R (number of Resonance Structures) please draw all the resonance structures. Make sure you keep track of all formal charges and lone pairs. A: Proton (a) is more acidic because of A (atom type and/or size in ARIO) B: Proton (b) is more acidic because of A (atom type and/or size in ARIO) C: Proton (a) is more acidic because of R (number of resonance structures in ARIO) D: Proton (b) is more acidic because of R (number of resonance structures in ARIO) E: Proton (a) is more acidic because of I (induction in ARIO) F: Proton (b) is more acidic because of I (induction in ARIO) G: Protoin (a) is more acidic because of O (orbital type in ARIO) H: Proton (b) is more acidic because of O (orbital type in ARIO) For the compound below please determine which of the two protons (a) or (b) is more acidic using the ARIO rule and explain your reasoning. Note: To answer the question correctly, please draw both Conjugate Base 1 and Conjugate Base 2. Determine which of the two protons is more acidic using the ARIO rule and explain your reasoning - if the reason is R (number of Resonance Structures) please draw all the resonance structures. Make sure you keep track of all formal charges and lone pairs. A: Proton (a) is more acidic because of A (atom type and/or size in ARIO) B: Proton (b) is more acidic because of A (atom type and/or size in ARIO) C: Proton (a) is more acidic because of R (number of resonance structures in ARIO) D: Proton (b) is more acidic because of R (number of resonance structures in ARIO) E: Proton (a) is more acidic because of I (induction in ARIO) F: Proton (b) is more acidic because of I (induction in ARIO) G: Proton (a) is more acidic because of O (orbital type in ARIO) H : Proton (b) is more acidic because of O (orbital type in ARIO) The structures below are two resonance structures of benzene: True False
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


