Question: Please, if you know the answer, let me know! 2. Butane (C4H10, MW 58.1) and n-propanol (C3H8O,MW60.1) have similar molecular weights, yet their boiling points
Please, if you know the answer, let me know!
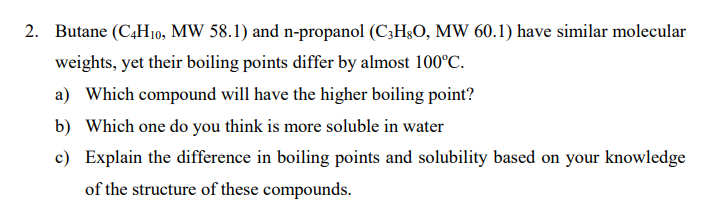
2. Butane (C4H10, MW 58.1) and n-propanol (C3H8O,MW60.1) have similar molecular weights, yet their boiling points differ by almost 100C. a) Which compound will have the higher boiling point? b) Which one do you think is more soluble in water c) Explain the difference in boiling points and solubility based on your knowledge of the structure of these compounds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


