Question: please ignore that first picture. i need help with this one. Rate=k[Cl][I2]2[ClO]Rate=[Cl]k[L][ClO]Rate=k[I[[ClO][Cl]Rate=k[Cl][[][CiO1]2 Correct By comparing Experiments 2 and 3 , you see that doubling [I
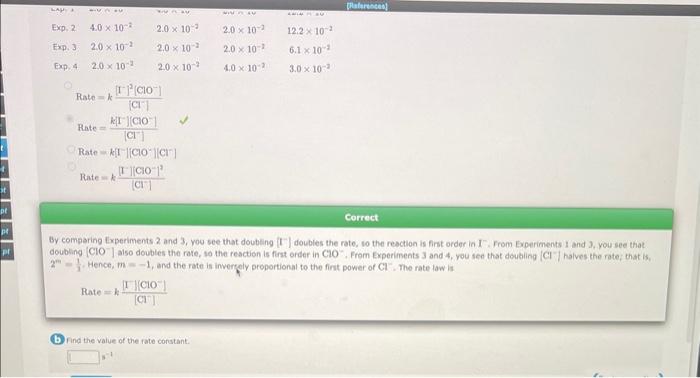
![Rate=k[Cl][I2]2[ClO]Rate=[Cl]k[L][ClO]Rate=k[I[[ClO][Cl]Rate=k[Cl][[][CiO1]2 Correct By comparing Experiments 2 and 3 , you see that](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d77984147_12166f8d779380cc.jpg)
Rate=k[Cl][I2]2[ClO]Rate=[Cl]k[L][ClO]Rate=k[I[[ClO][Cl]Rate=k[Cl][[][CiO1]2 Correct By comparing Experiments 2 and 3 , you see that doubling [I | doubles the rate, to the resction is fint order in I. From Experiments I and 3 , you see that doubling [ClO]also doubles the rete, so the reaction is first order in ClO. From Experiments 3 and 4 , you see that doubling [Clhalves the rate; that is 2m=11. Hence, m=1, and the rate is invergely proportional to the fint power of Cl. The rate law is Rate=k[Cl][I][ClO] (b) find the value of the rate corstant. CH3CH2Cl(g)C2H4(g)+HCl(g) The reaction is first orden. in an experiment, the inital concentration of ethyl chioride was 1.00107M. Afer heating at 500C for 155 s, this was reduced to 6.70101M. What was the concentration of ethyl chloride after a total of 216s ? Concentration = M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


