Question: Please include a step-by-step breakdown. thanks. 2. Balance the following redox reactions. (10 marks) a. IO31 (aq) +SO32 (aq) I2 (aq) +SO42 (aq) b. MnO41(aq)+C2H5OH(1)Mn+2(aq)+C2H4O2
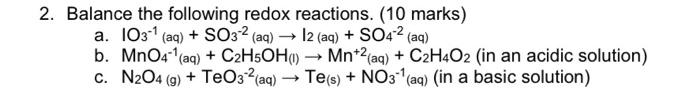
2. Balance the following redox reactions. (10 marks) a. IO31 (aq) +SO32 (aq) I2 (aq) +SO42 (aq) b. MnO41(aq)+C2H5OH(1)Mn+2(aq)+C2H4O2 (in an acidic solution) c. N2O4(g)+TeO32(aq)Te(s)+NO31 (aq) (in a basic solution)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


