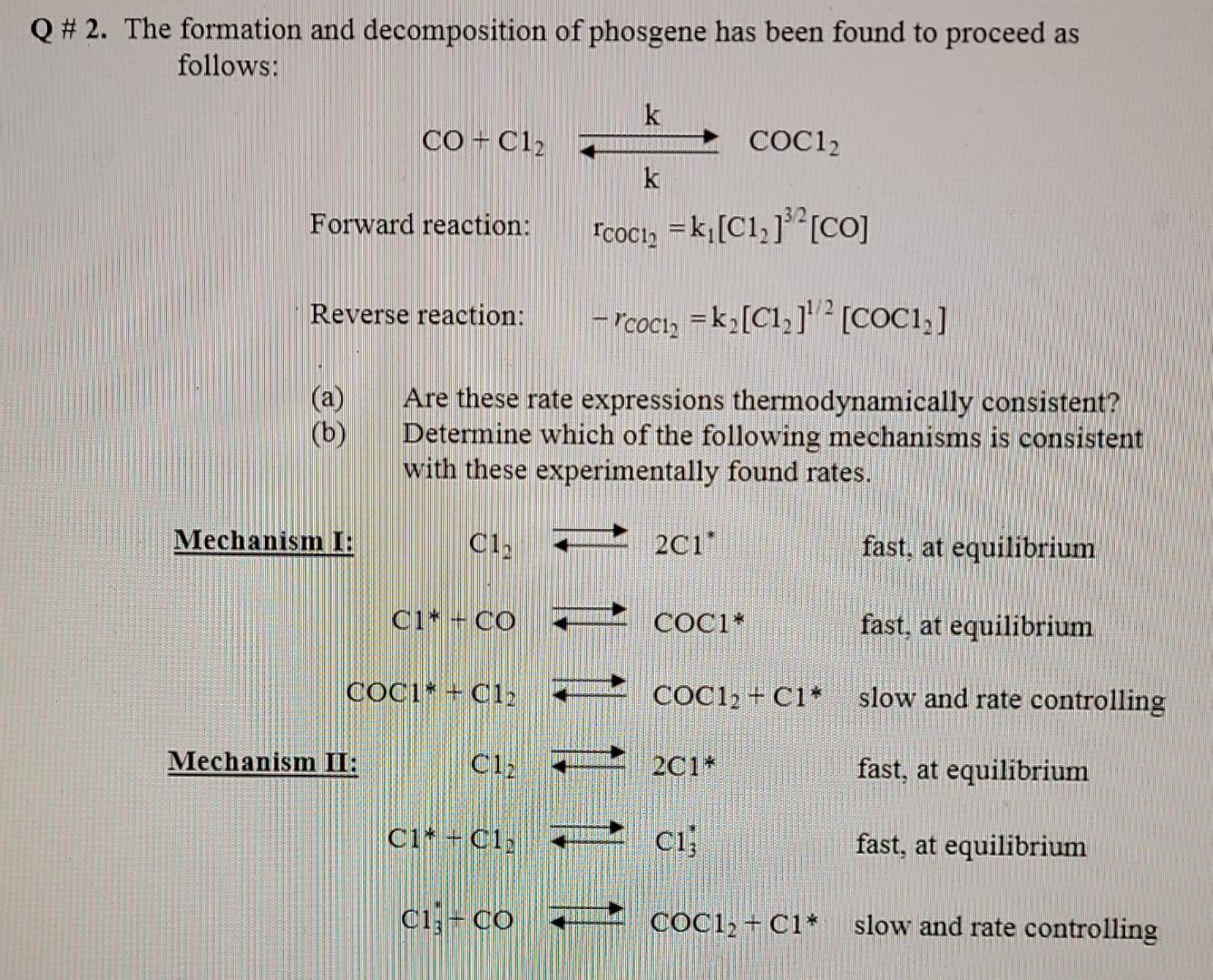
Question: Please include as much detail in your answer as possible. Thanks Q# 2. The formation and decomposition of phosgene has been found to proceed as
Please include as much detail in your answer as possible. Thanks
Q# 2. The formation and decomposition of phosgene has been found to proceed as follows: k CO + C12 COC12 k Forward reaction: "Coci2 = k (C1,}}? [CO] Reverse reaction: Hrcoci, = k[C1,]'? [COC1, ] (b) Are these rate expressions thermodynamically consistent? Determine which of the following mechanisms is consistent with these experimentally found rates. Mechanism I! ci, 201" fast, at equilibrium C1* CO LLLLL COC1* fast, at equilibrium COCIN + 012 LILLE COC12 + Ci* slow and rate controlling Mechanism II: Cla 201* fast, at equilibrium C11 +61) ci; fast, at equilibrium clico coc12 + C1* slow and rate controlling
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


