Question: Please include steps and explanations, thank you. 3. Balance the following redox reactions by the half-reaction method. Identify the half oxidation and reduction reactions. The
Please include steps and explanations, thank you.
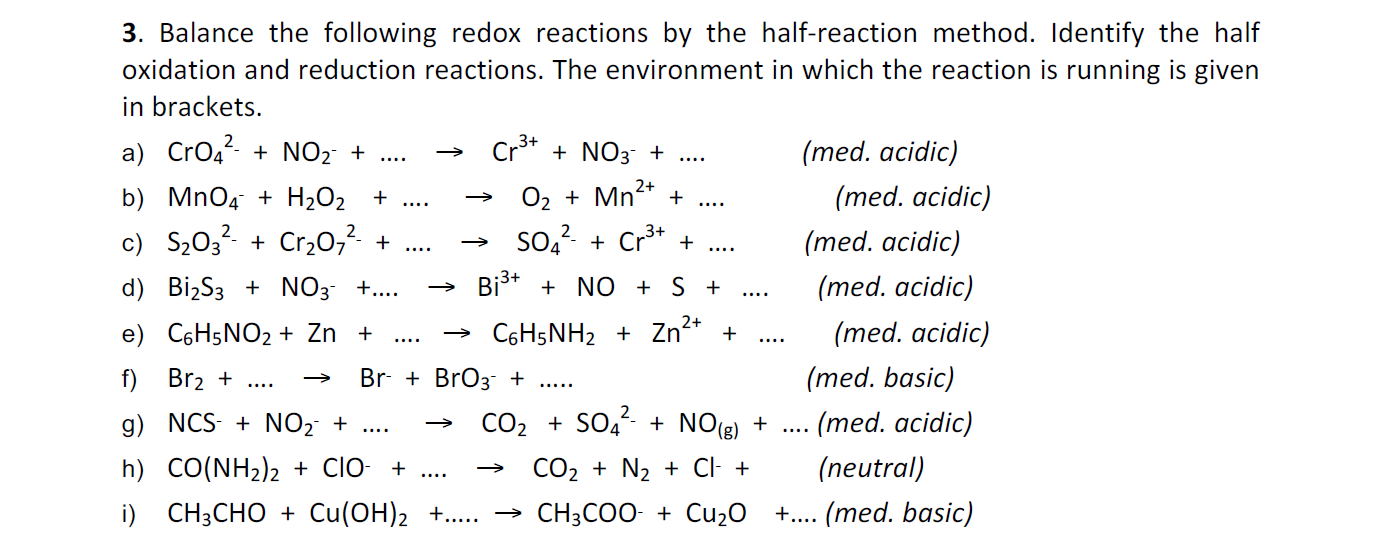
3. Balance the following redox reactions by the half-reaction method. Identify the half oxidation and reduction reactions. The environment in which the reaction is running is given in brackets. a) CrO42NO2+.Cr3++NO3+. (med. acidic) b) MnO4+H2O2+.O2+Mn2++. (med. acidic) c) S2O32+Cr2O72+.SO42Cr3++. (med. acidic) d) Bi2S3+NO3+.Bi3++NO+S+. (med. acidic) e) C6H5NO2+Zn+C6H5NH2+Zn2++ (med. acidic) f) Br2+.Br+BrO3+.. (med. basic) g) NCSNO2+.CO2+SO42+NO(g)+. (med. acidic) h) CO(NH2)2+ClO+.CO2+N2+Cl+(neutral) i) CH3CHO+Cu(OH)2+..CH3COO+Cu2O+. (med. basic)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


