Question: Please include steps and formulas used for calculations. Please provide the readable solution if you're going to provide handwriting solutions. Thank you. 2. What is
Please include steps and formulas used for calculations. Please provide the readable solution if you're going to provide handwriting solutions. Thank you.
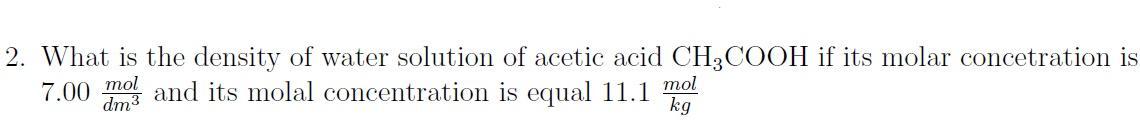
2. What is the density of water solution of acetic acid CH3COOH if its molar concetration is 7.00dm3mol and its molal concentration is equal 11.1kgmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


