Question: please just explain e, f and g 2A+BC is carried out isothermally in a PFR with no pressure drop. The feed is equal molar in
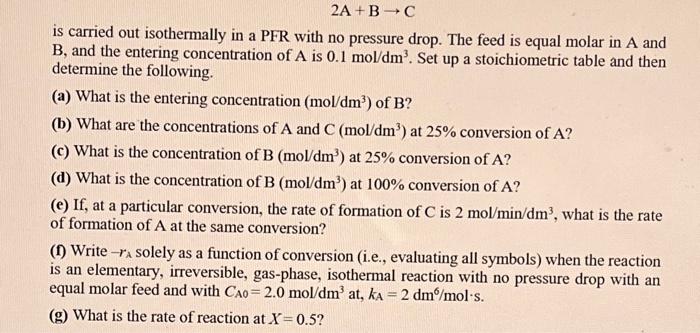
2A+BC is carried out isothermally in a PFR with no pressure drop. The feed is equal molar in A and B, and the entering concentration of A is 0.1mol/dm3. Set up a stoichiometric table and then determine the following. (a) What is the entering concentration (mol/dm3) of B ? (b) What are the concentrations of A and C(mol/dm3) at 25% conversion of A ? (c) What is the concentration of B(mol/dm3) at 25% conversion of A ? (d) What is the concentration of B(mol/dm3) at 100% conversion of A ? (e) If, at a particular conversion, the rate of formation of C is 2mol/min/dm3, what is the rate of formation of A at the same conversion? (f) Write rA solely as a function of conversion (i.e., evaluating all symbols) when the reaction is an elementary, irreversible, gas-phase, isothermal reaction with no pressure drop with an equal molar feed and with CA0=2.0mol/dm3at,kA=2dm6/mols. (g) What is the rate of reaction at X=0.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


