Question: * PLEASE JUST SOLVE PARTS E , F , G , H The system to be studied in this problem has an average enthalpy of
PLEASE JUST SOLVE PARTS EFGH
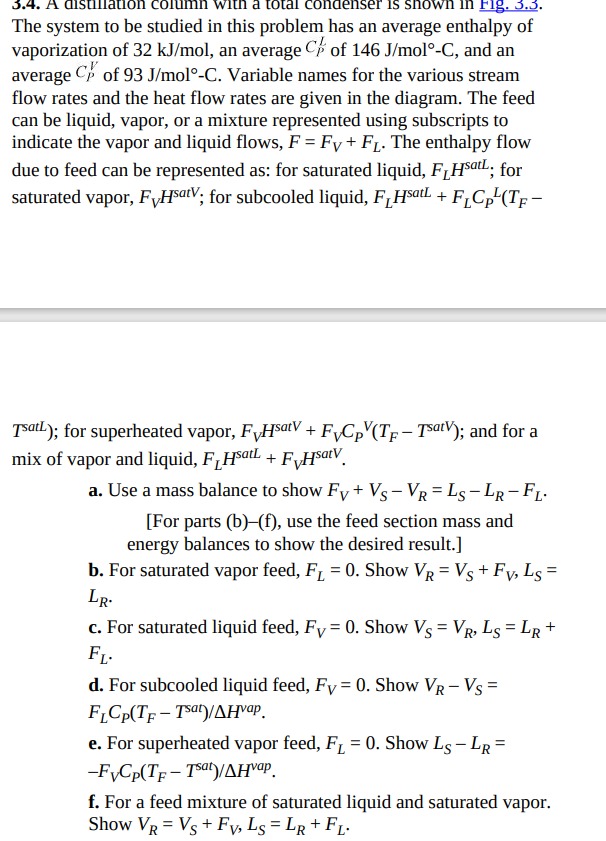
The system to be studied in this problem has an average enthalpy of
vaporization of an average of and an
average of C Variable names for the various stream
flow rates and the heat flow rates are given in the diagram. The feed
can be liquid, vapor, or a mixture represented using subscripts to
indicate the vapor and liquid flows, The enthalpy flow
due to feed can be represented as: for saturated liquid, ; for
saturated vapor, ; for subcooled liquid,
:; for superheated vapor, ; and for a
mix of vapor and liquid,
a Use a mass balance to show
For parts bf use the feed section mass and
energy balances to show the desired result.
b For saturated vapor feed, Show
c For saturated liquid feed, Show
d For subcooled liquid feed, Show
e For superheated vapor feed, Show
f For a feed mixture of saturated liquid and saturated vapor.
Show
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


