Question: please just write the answer don't write me your life story I don't need any explanation The manufacturer of the vinegar used in this experiment


 please just write the answer don't write me your life story I don't need any explanation
please just write the answer don't write me your life story I don't need any explanation
The manufacturer of the vinegar used in this experiment claims that the vinegar contains 5 % acetic acid by weight . Use your results and a density of 1.0 g /mL to investigate this claim?
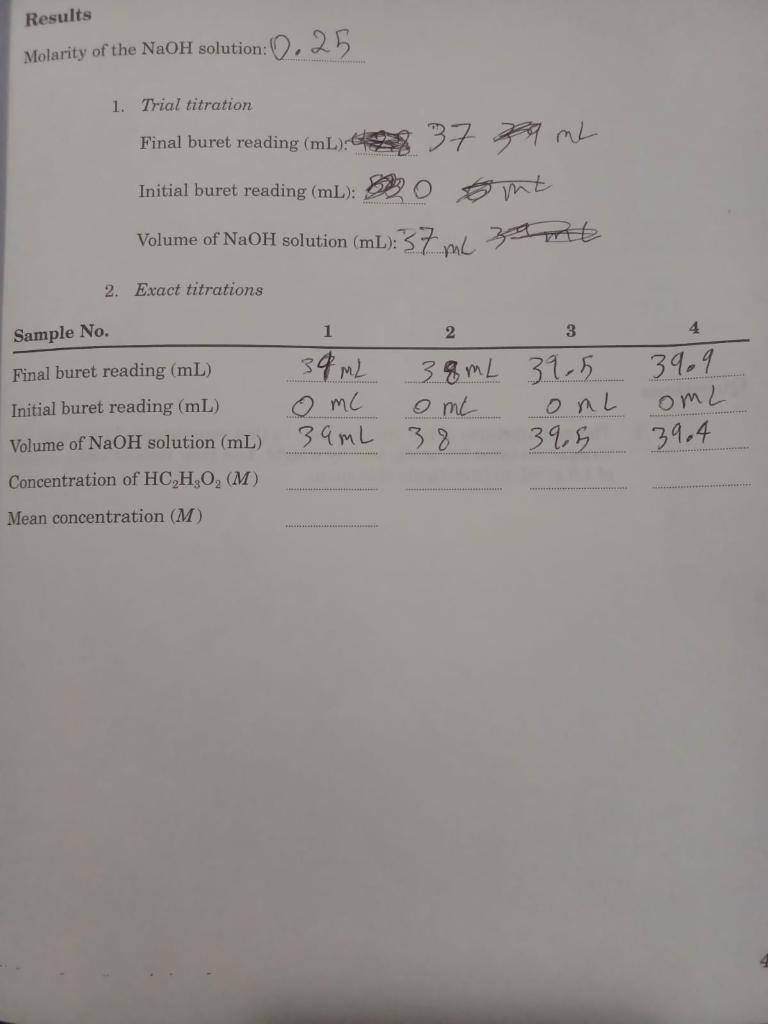
5. How Much Acetic Acid Is in Vinegar? Introduction Quantitative analysis determines the amount of a particular substance in a sample. Often, this determination is accomplished through a titration (Ebbing/Gammon, Section 4.10) of the sample with a solution of another sub- stance whose concentration is known. These substances must react with each other, of course. Some physical or chemical response must be coupled to the titration to signal the exact completion of the reaction. An indicator can sometimes be used. The indicator chosen will have one color before the reaction is complete and an- other color when completion occurs. After the indicator is added to the sample solution, the solution of known concentration is delivered carefully from a buret until the indicator changes color. The Introduction to this manual de scribes the proper use of a buret. Purpose You will determine the amount of acetic acid in white vinegar by titration with a solution of sodium hydroxide whose concentration is known. The indicator will be phenolphthalein. Concept of the experiment The object of this experiment is to determine the molar concentration (Ebbing Gammon, Section 4.7) of acetic acid (HC,H,O,) in vinegar. You will accomplish this through the titration of a sample of vinegar with a solution of sodium hydroxide (NaOH). These substances react readily HC,H,O, + NaOH-NaC,H,O, + H2O Phenolphthalein will be used as the indicator. It will be colorless before the completion of this reaction but pink after the completion. You must be pre- pared to search carefully for a point in the titration at which 1 drop of the NaOH solution causes the solution being titrated to turn from colorless to a barely discernible pink color. This point is called the endpoint. You will do a trial titration to find the approximate endpoint before you do a pair of exact titrations. These titrations, the molarity of the solution of sodium hydroxide and the balanced equation, will provide all that you need to determine the molarity of the acetic acid. Procedure Getting started 1. Obtain a 10-ml transfer pipet and a 50-ml buret 2. Next obtain about 30 ml of white 5% vinegar and about 85 mL of the solu- tion of NaOH. The vinegar may be kept in a clean, dry beaker. 37 However, the NaOH solution must be kept in a clean, dry Erlenmeyer flask that is closed with a rubber stopper: This solution must be protected when it is in use because NaOH will react with carbon dioxide (CO) in the air. The larity of this solution will lie between 0.2 M and 0.3 M; the exact concentration will be given on the label of the bottle. How Much A 03/2 Date: Chem Section: Instructor Results Cleaning and filling your buret Course 1. The Introduction to this manual gives instructions for using a buret. Cleme your buret and fill it with the NaOH solution after you have read those pro cedures carefully Doing the trial titration 1. Pipet 10.0 ml. of vinegar into a clean 125-ml. Erlenmeyer flask. Add abou Molarity of the Nac 20 mL of distilled water from a clean graduated cylinder. Add 2 drops phenolphthalein solution CAUTION: Never use your mouth to draw liq- uid into the pipet. Use a rubber suction bulb or some other suction device. 1. THE Fir Ini Record the molarity of the NaOH solution and the initial buret reading. Vo 3. Place the flask under the buret with the capillary tip inside the mouth the flask. Place a piece of white paper under the flask. 2. ES 4. Add the NaOH solution to the flask in increments of about 1 mL whij swirling. Note the color of the solution after each addition 5. This trial titration is complete when an addition of about 1 mL causes the Sample No. color to change from colorless to any shade of pink. Final buret readi 6. Record the buret reading. Subtract the initial reading from this reading Initial buret read obtain the volume required for the approximate endpoint. Volume of NaOH Doing the exact titrations Concentration of 1. Repeat Steps 1, 2, and 3 of the procedure used for the trial titration 2. Subtract 1 ml from the volume found in the trial titration. Rapidly add ti Mean concentrat resulting volume to the flask from the buret. 3. Rinse the walls of the flask with distilled water from a plastic wash bottl 4. Continue the titration on a drop-by-drop basis. Swirl the flask rapidly afte each drop. The endpoint is the first permanent, barely visible pink coli Finding the true endpoint requires patience and skill. Absolutely no skill required to miss the endpoint and achieve a very deep pink color. If you unsure about the endpoint, record the buret reading before you add t next drop 5. Repeat the procedure with a second sample of vinegar. 6. If the volumes at the endpoints of these two exact titrations differ by mom than 0.15 mL (about 3 drops), repeat the titrations with additional samp of vinegar until two consecutive results have this precision 7. Calculate and record the molarity of the vinegar from each of the two tim tions. Does each molarity have the correct number of significant figum Obtain the mean molarity Results Molarity of the NaOH solution: 0.25 1. Trial titration Final buret reading (mL): 37 9 mL mt Volume of NaOH solution (ml): 37 ml samt Initial buret reading (mL): 2. Exact titrations 1 Sample No. 2 3 4 39 m2 Final buret reading (mL) Initial buret reading (mL) Volume of NaOH solution (mL) 38mL 39.5 o me 39mL 38 39,5 o mc 39.9 onl omL 39.4 Concentration of HC,H,O, (M) Mean concentration (M)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


