Question: Please let me know if I got anything wrong in 3. Otherwise I just need help with 4 as I do not understands the IMFs
Please let me know if I got anything wrong in 3. Otherwise I just need help with 4 as I do not understands the IMFs associated with a solute-solute interaction, a solute-solvent interaction, and a solvent-solvent interaction.
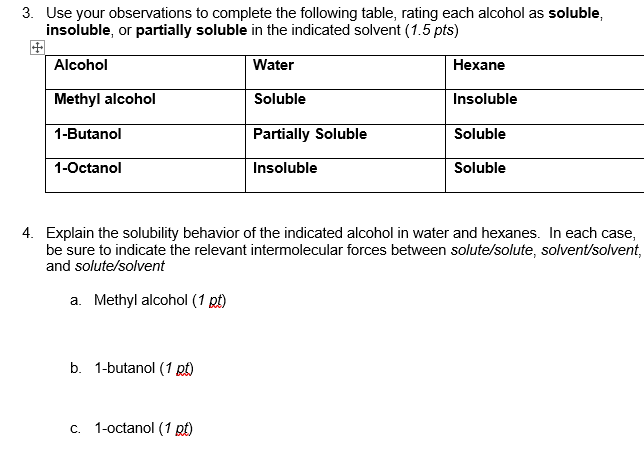
3. Use your observations to complete the following table, rating each alcohol as soluble, insoluble, or partially soluble in the indicated solvent (1.5 pts) + Alcohol Water Hexane Methyl alcohol Soluble Insoluble 1-Butanol Partially Soluble Soluble 1-Octanol Insoluble Soluble 4. Explain the solubility behavior of the indicated alcohol in water and hexanes. In each case, be sure to indicate the relevant intermolecular forces between solute/solute, solvent/solvent, and solute/solvent a. Methyl alcohol (1 pt) b. 1-butanol (1 t) C. 1-octanol (1 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


