Question: please look at the note below before solving Q1. Vapor Pressure and boiling (15 marks) At approximately what temperature will water boil on top of
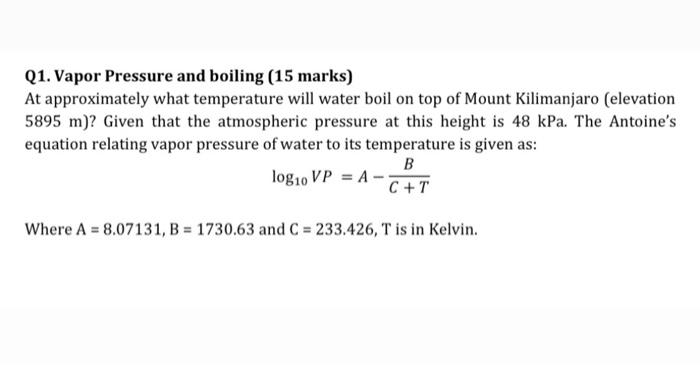

Q1. Vapor Pressure and boiling (15 marks) At approximately what temperature will water boil on top of Mount Kilimanjaro (elevation 5895 m)? Given that the atmospheric pressure at this height is 48 kPa. The Antoine's equation relating vapor pressure of water to its temperature is given as: B log10 VP = A - C + T Where A = 8.07131, B = 1730.63 and C = 233.426, T is in Kelvin. Please consider that T is in Celcius and Vapor Pressure is in mm Hg or torr. In the question it states that T is in Kelvin and does not give a unit for VP, 1 apologize for this mistake
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


