Question: please make sure your answer is clearly written and % correct 13. A hydrogen like ion is an ion containing only one electron. The energies
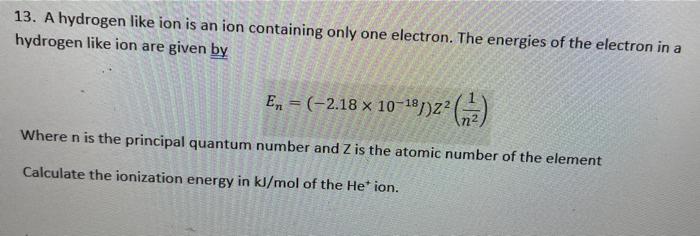
13. A hydrogen like ion is an ion containing only one electron. The energies of the electron in a hydrogen like ion are given by En=(2.181018J)Z2(n21) Where n is the principal quantum number and Z is the atomic number of the element Calculate the ionization energy in kJ/mol of the He+ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


