Question: please need help with this ill thumbs up The following initial rate data are for the reaction of UO2 with hydrogen lon In aqueous solution:

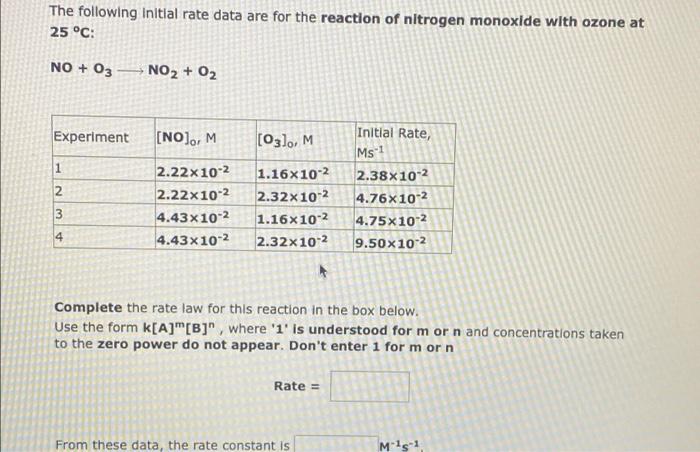
The following initial rate data are for the reaction of UO2 with hydrogen lon In aqueous solution: 2 102t + 4 H+ -U4+ + U022+ + 2 H20 Experiment (102), M (H+), M Initial Rate, M 1 s1 2 0.394 0.394 3 2.37x10-3 4.74x10-3 2.37x10-3 4.74x10-3 0.788 3.25x104 1.30x103 6.51x104 2.60x10" 4 0.788 Complete the rate law for this reaction in the box below. Use the form k[A]"[B]", where '1' is understood for mor n and concentrations taken to the zero power do not appear. Don't enter 1 for mor n. Rate = From these data, the rate constant is M?1 The following Initial rate data are for the reaction of nitrogen monoxide with ozone at 25 C: NO + 03 NO2 + O2 Experiment (NO), M (O3), M 1 2 2.22x 10-2 2.22x10-2 4.43x10-2 4.43x10-2 Initial Rate Ms 2.38x10-2 4.76X10-2 4.75x10-2 9.50x102 1.16x10-2 2.32x10-2 1.16x10-2 2.32x10-2 3 4 Complete the rate law for this reaction in the box below. Use the form k[A]"[B]", where '1' is understood for mor n and concentrations taken to the zero power do not appear. Don't enter 1 for mor n Rate = From these data, the rate constant is M15-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


