Question: please please i need help with these questions ASAP 2. Below is an ionisation energy graph created by removing the first valence electron from Woeld
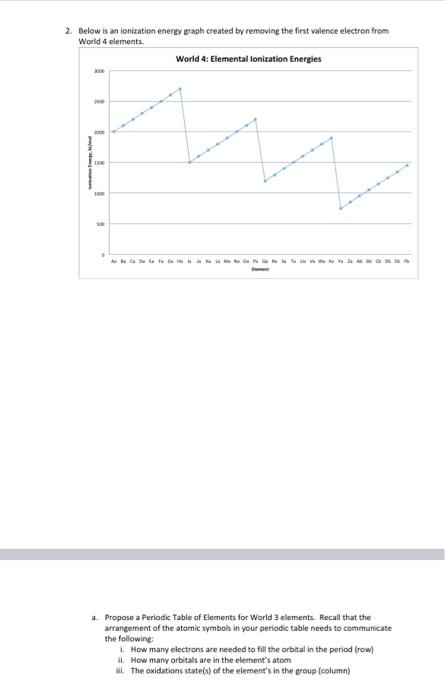


2. Below is an ionisation energy graph created by removing the first valence electron from Woeld 4 elements. a. Propose a Periodic Table of Elements for World 3 elements. Recall that the arrangement of the atomic symbols in your periodic table needs to communicate the follawing: i. How many electrons are needed to fill the orbital in the period (row) ii. How many orbitals are in the element's atom ii. The axidations state(s) of the element's in the group (column) c. Propose a Bohr model for element Oa. Label the nucleus and the valence electron(s). d. Propose a Bohr model for the element Oa after the valence electron has been danated. Label the nucleus and the valence electron(s) has/have been removed. e. What is the oxidation state of the Oa ion? f. Explain how looking at Bohr models of the Da atom and the Oa ion you drew in parts c and d demonstrates the oxidation state for the group in the periodic table you propesed in part a. b. Indicate the oxidation state(s) for the groups in your periodic table by writing them above the column for the group. 2. Below is an ionisation energy graph created by removing the first valence electron from Woeld 4 elements. a. Propose a Periodic Table of Elements for World 3 elements. Recall that the arrangement of the atomic symbols in your periodic table needs to communicate the follawing: i. How many electrons are needed to fill the orbital in the period (row) ii. How many orbitals are in the element's atom ii. The axidations state(s) of the element's in the group (column) c. Propose a Bohr model for element Oa. Label the nucleus and the valence electron(s). d. Propose a Bohr model for the element Oa after the valence electron has been danated. Label the nucleus and the valence electron(s) has/have been removed. e. What is the oxidation state of the Oa ion? f. Explain how looking at Bohr models of the Da atom and the Oa ion you drew in parts c and d demonstrates the oxidation state for the group in the periodic table you propesed in part a. b. Indicate the oxidation state(s) for the groups in your periodic table by writing them above the column for the group
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


