Question: Please plot in Polymath. P6-118 Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol.
Please plot in Polymath.
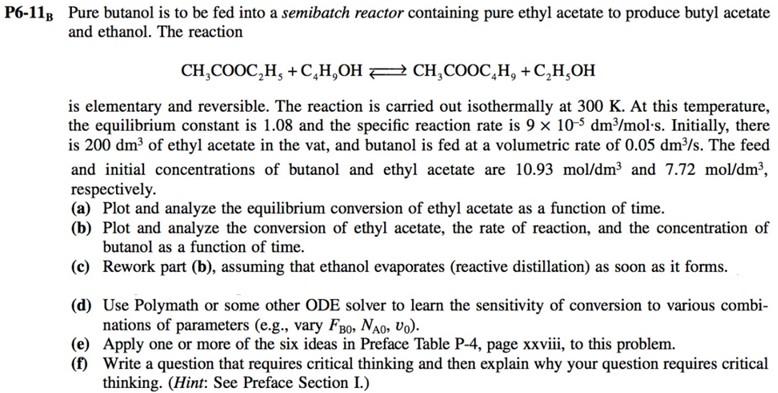
P6-118 Pure butanol is to be fed into a semibatch reactor containing pure ethyl acetate to produce butyl acetate and ethanol. The reaction CH,COOC,H, +C,H,OH PCH,COOC,H, +C,H,OH is elementary and reversible. The reaction is carried out isothermally at 300 K. At this temperature, the equilibrium constant is 1.08 and the specific reaction rate is 9 x 10-5 dm3/mol-s. Initially, there is 200 dm3 of ethyl acetate in the vat, and butanol is fed at a volumetric rate of 0.05 dm3/s. The feed and initial concentrations of butanol and ethyl acetate are 10.93 mol/dm3 and 7.72 mol/dm?, respectively. (a) Plot and analyze the equilibrium conversion of ethyl acetate as a function of time. (b) Plot and analyze the conversion of ethyl acetate, the rate of reaction, and the concentration of butanol as a function of time. (c) Rework part (b), assuming that ethanol evaporates (reactive distillation) as soon as it forms. (d) Use Polymath or some other ODE solver to learn the sensitivity of conversion to various combi- nations of parameters (e.g., vary FBO, NO, Vo). (e) Apply one or more of the six ideas in Preface Table P-4, page xxviii, to this problem. (1) Write a question that requires critical thinking and then explain why your question requires critical thinking. (Hint: See Preface Section I.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


