Question: please post step by step solution Susan found a cylinder of CO2 in the lab and claims it contains a liquid-vapour mixture of pure CO2.
please post step by step solution
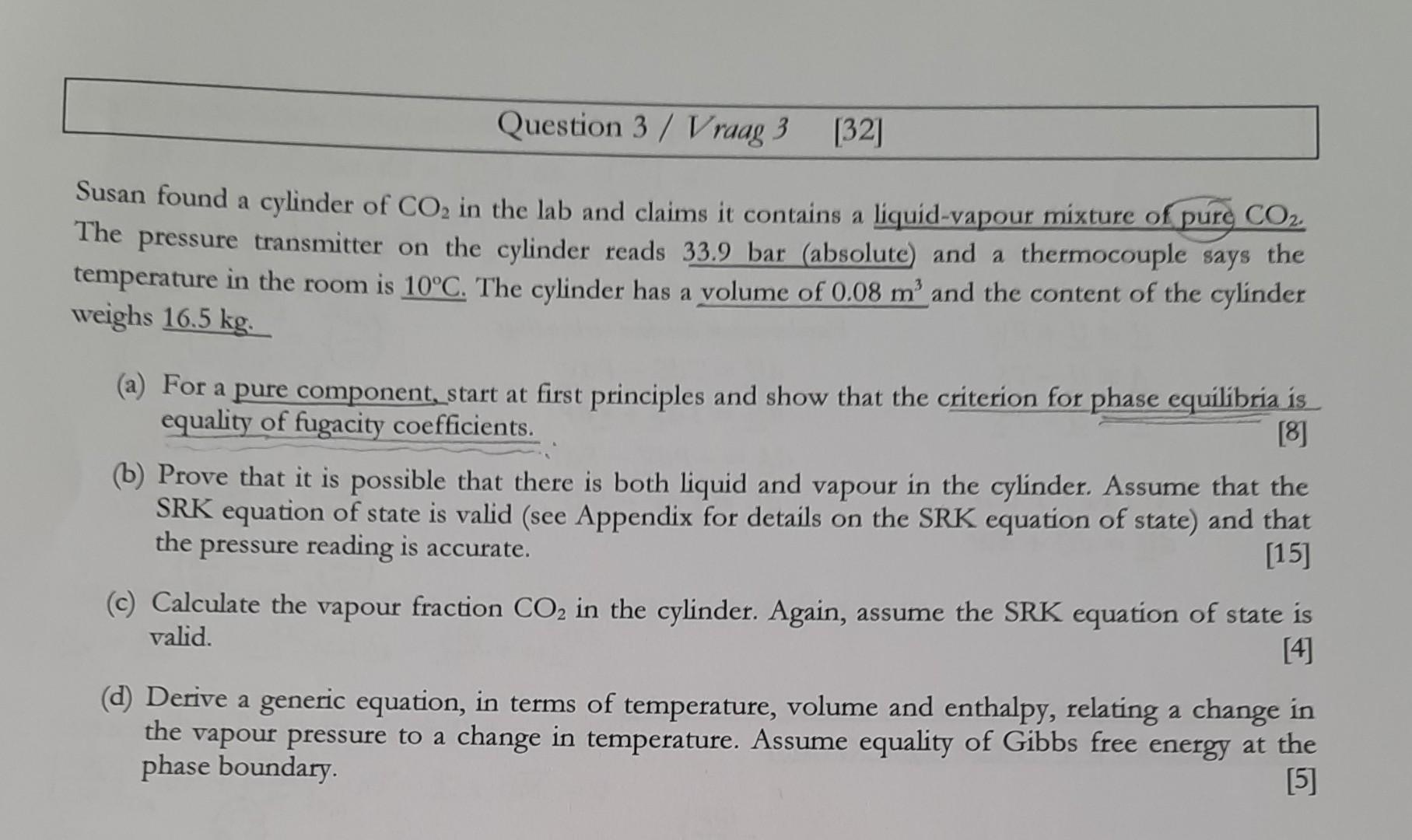
Susan found a cylinder of CO2 in the lab and claims it contains a liquid-vapour mixture of pure CO2. The pressure transmitter on the cylinder reads 33.9bar (absolute) and a thermocouple says the temperature in the room is 10C. The cylinder has a volume of 0.08m3 and the content of the cylinder weighs 16.5kg. (a) For a pure component, start at first principles and show that the criterion for phase equilibria is equality of fugacity coefficients. (b) Prove that it is possible that there is both liquid and vapour in the cylinder. Assume that the SRK equation of state is valid (see Appendix for details on the SRK equation of state) and that the pressure reading is accurate. [15] (c) Calculate the vapour fraction CO2 in the cylinder. Again, assume the SRK equation of state is valid. (d) Derive a generic equation, in terms of temperature, volume and enthalpy, relating a change in the vapour pressure to a change in temperature. Assume equality of Gibbs free energy at the phase boundary. [5]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


