Question: Please provide a step by step answer to this (7%) Problem 5: A 97.4-g aluminum calorimeter contains 250 g of water. The aluminum and water
Please provide a step by step answer to this
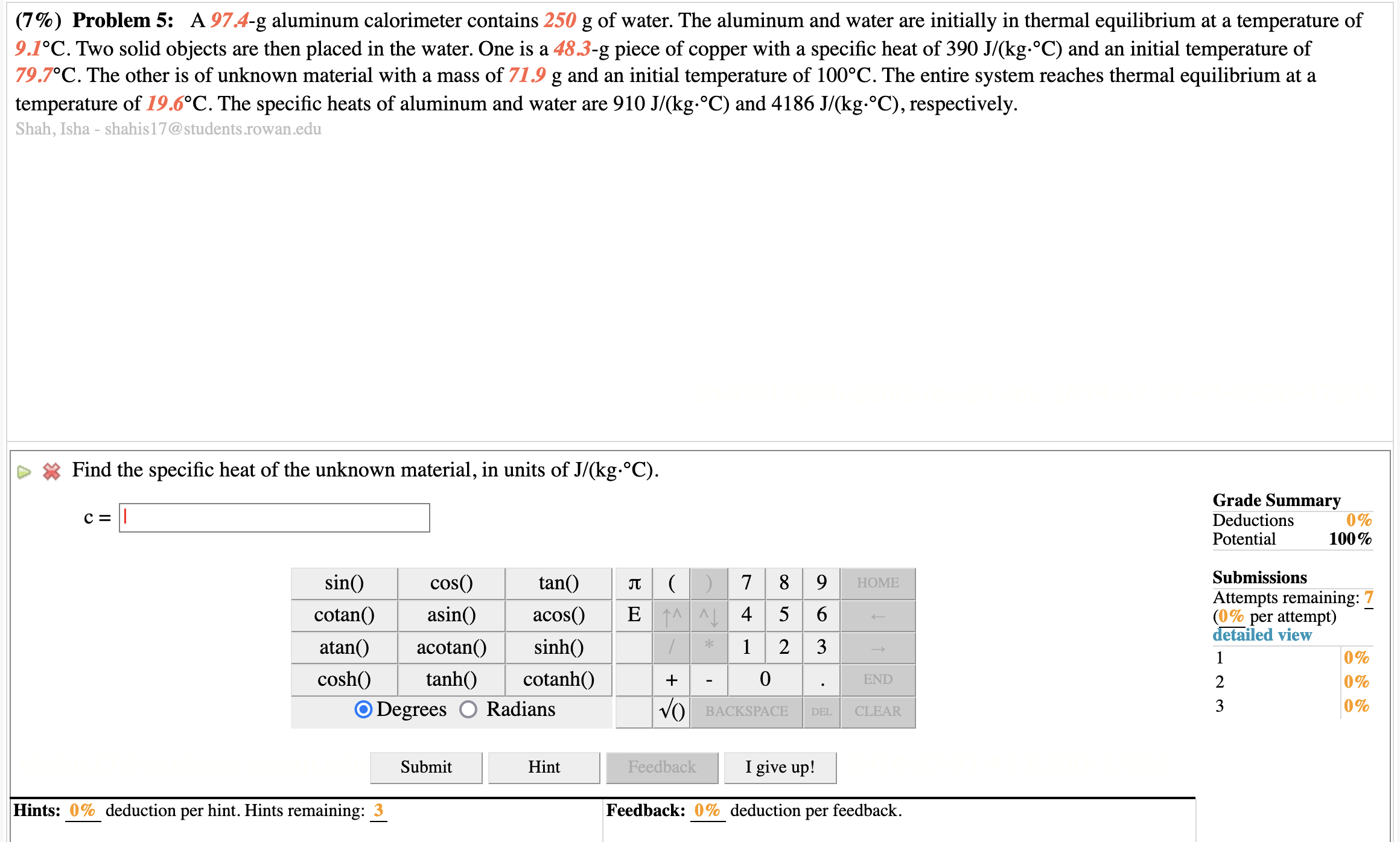
(7%) Problem 5: A 97.4-g aluminum calorimeter contains 250 g of water. The aluminum and water are initially in thermal equilibrium at a temperature of 9.1.C. Two solid objects are then placed in the water. One is a 48.3-g piece of copper with a specific heat of 390 J/(kg. C) and an initial temperature of 79.7.C. The other is of unknown material with a mass of 71.9 g and an initial temperature of 100 C. The entire system reaches thermal equilibrium at a temperature of 19.6 C. The specific heats of aluminum and water are 910 J/(kg. C) and 4186 J/(kg. C), respectively. Shah, Isha - shahis17@students.rowan.edu Find the specific heat of the unknown material, in units of J/(kg. C). Grade Summary C= Deductions 0% Potential 100% sin() cos() tan( 7 8 9 HOME Submissions Attempts remaining: 7 cotan() asin() acos() E A 4 5 6 (0% per attempt detailed view atan() acotan() sinh() 2 3 1 0% cosh() tanh() cotanh() + 0 END % WN O Degrees O Radians VO BACKSPACE DEL CLEAR 0% Submit Hint Feedback I give up! Hints: 0% deduction per hint. Hints remaining: 3 Feedback: 0% deduction per feedback
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


