Question: please provide all possible steps, thanks for question 1 i found that wor B=917.43(bottom product) and for D top product D -317 Q1 A saturated
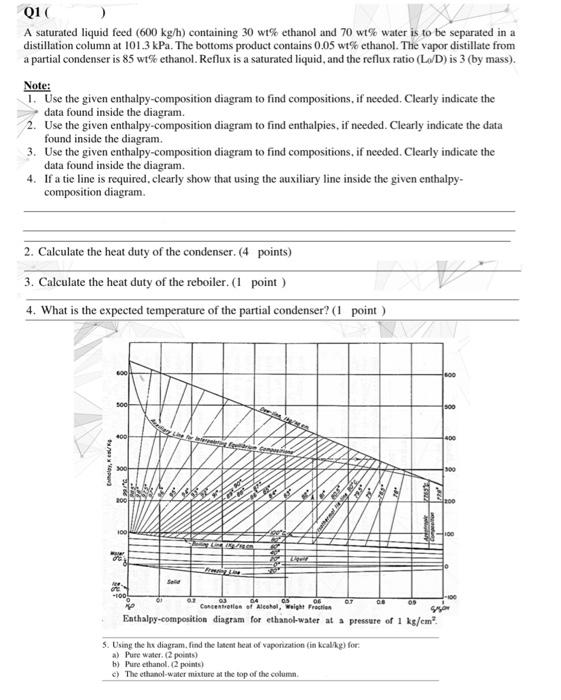

Q1 A saturated liquid feed (600 kg/h) containing 30 wt% ethanol and 70 wt% water is to be separated in a distillation column at 101.3 kPa. The bottoms product contains 0.05 wt% ethanol. The vapor distillate from a partial condenser is 85 wt% ethanol. Reflux is a saturated liquid, and the reflux ratio (L/D) is 3 (by mass). Note: 1. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram. 2. Use the given enthalpy-composition diagram to find enthalpies, if needed. Clearly indicate the data found inside the diagram. 3. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram. 4. If a tie line is required, clearly show that using the auxiliary line inside the given enthalpy- composition diagram 2. Calculate the heat duty of the condenser. (4 points) V 3. Calculate the heat duty of the reboiler. (1 point) 4. What is the expected temperature of the partial condenser? (1 point) 600 300 400 ENKEK 100 BESONA 100 -100 05 06 0.1 04 Concentration of Alcohol, where Enthalpy-composition diagram for ethanol-water at a pressure of 1 ks/em 5. Using the hx diagram, find the latent heat of vaporization (in kcal/kg) for a) Pure Water (2 points) b) Preethanol. 2 points) c) The ethanol-water mixture at the top of the column Q1 A saturated liquid feed (600 kg/h) containing 30 w ethanol and 70 wtwater is to be separated in a distillation column at 101.3 kPa. The bottoms product contains 0.05 wt% ethanol. The vapor distillate from a partial condenser is 85 wt% ethanol. Reflux is a saturated liquid, and the reflux ratio (LID) is 3 (by mass). Note: 1. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram 2. Use the given enthalpy-composition diagram to find enthalpies, if needed. Clearly indicate the data found inside the diagram. 3. Use the given enthalpy-composition diagram to find compositions, if needed. Clearly indicate the data found inside the diagram. 4. If a tie line is required, clearly show that using the auxiliary line inside the given enthalpy- composition diagram 1. Calculate the flow rates of the top and bottom products. (1 point 2. Calculate the heat duty of the condenser (4 points) 3. Calculate the heat duty of the reboiler. (1 point) 4. What is the expected temperature of the partial condenser?( point) ta Enthalpy-composition diagram for the pressure of 1 kg/em? 5. Ung the has drum.file oferite for Pure water print) Pure that point c) The esanol water in the top of the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


