Question: Please provide an explanation for your answer! True or false: If a material is produced in a reaction, the material balance equation will have a

 Please provide an explanation for your answer!
Please provide an explanation for your answer!
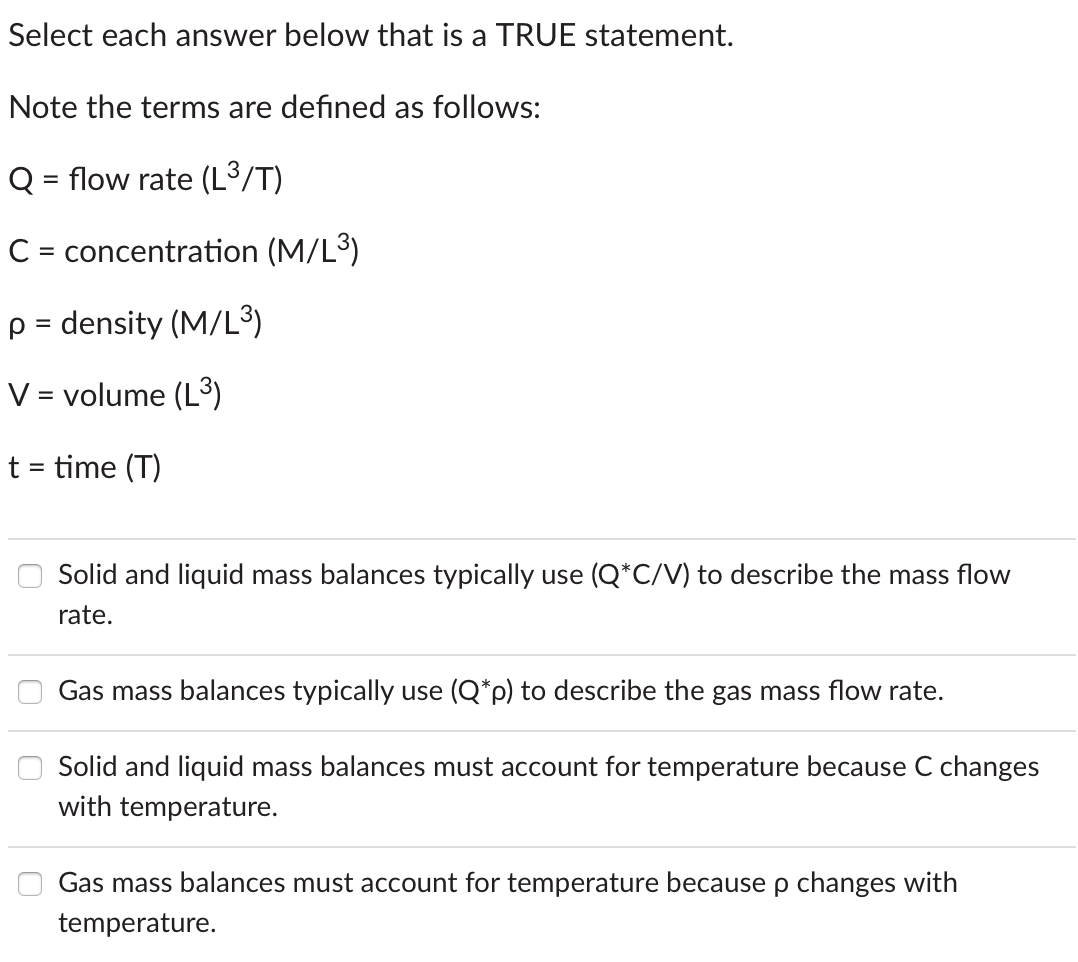
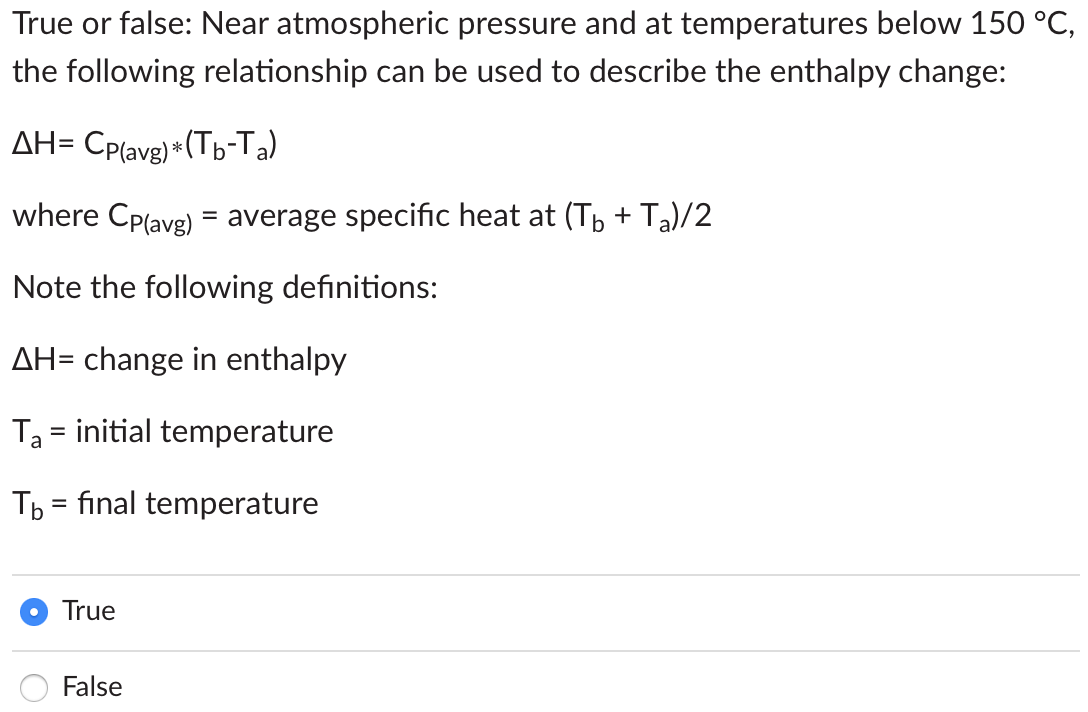
True or false: If a material is produced in a reaction, the material balance equation will have a positive Net Generation rate. True False Q=flowrate(L3/T)C=concentration(M/L3)=density(M/L3)V=volume(L3)t=time(T) Solid and liquid mass balances typically use (QC/V) to describe the mass flow rate. Gas mass balances typically use (Q) to describe the gas mass flow rate. Solid and liquid mass balances must account for temperature because C changes with temperature. Gas mass balances must account for temperature because changes with temperature. True or false: Near atmospheric pressure and at temperatures below 150C, the following relationship can be used to describe the enthalpy change: H=CP(avg)(TbTa) where CP(avg)= average specific heat at (Tb+Ta)/2 Note the following definitions: H= change in enthalpy Ta= initial temperature Tb= final temperature True False
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


