Question: Please provide an explanation on how you get this answer, An organic compound was found to contain only C,H, and Cl. When a 1.50g sample
Please provide an explanation on how you get this answer, 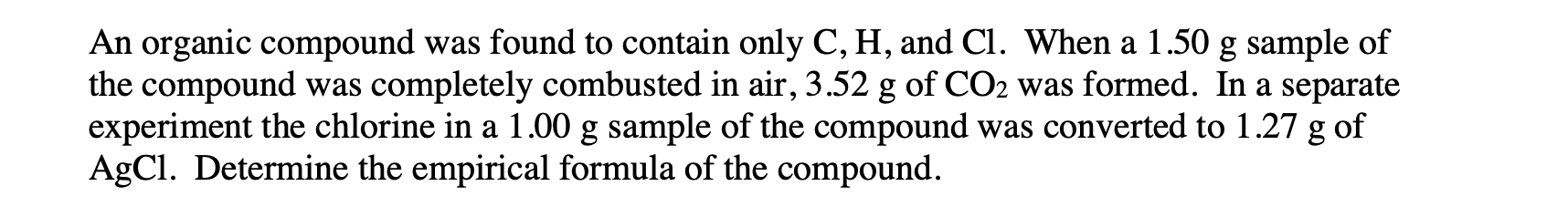
An organic compound was found to contain only C,H, and Cl. When a 1.50g sample of the compound was completely combusted in air, 3.52g of CO2 was formed. In a separate experiment the chlorine in a 1.00g sample of the compound was converted to 1.27g of AgCl. Determine the empirical formula of the compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


