Question: Please provide your answer with solution. Consider the following gas phase reaction: 051cd4d47dbb6c2F020b8d7913c7b5b6.png SO2(9)+30219) +50319) + Determine the amount of heat released for the complete
Please provide your answer with solution.
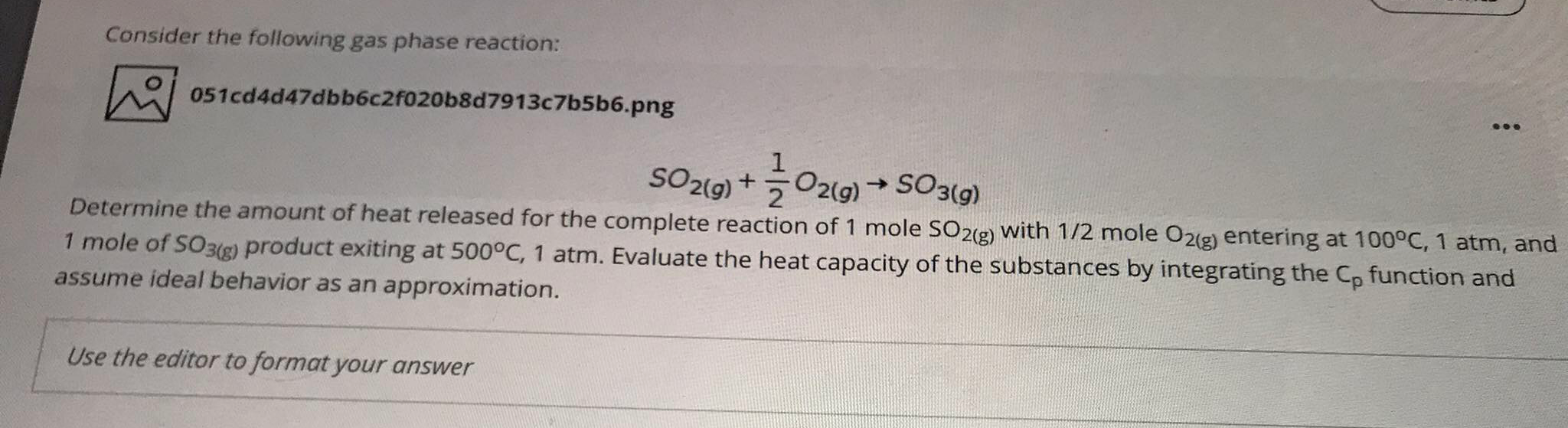
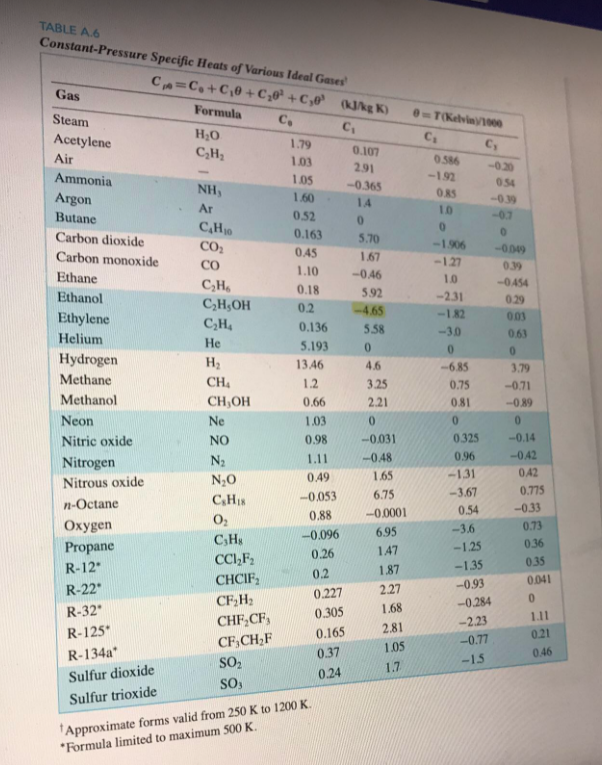
Consider the following gas phase reaction: 051cd4d47dbb6c2F020b8d7913c7b5b6.png SO2(9)+30219) +50319) + Determine the amount of heat released for the complete reaction of 1 mole SO2(g) with 1/2 mole O2(g) entering at 100C, 1 atm, and 1 mole of SO3(g) product exiting at 500C, 1 atm. Evaluate the heat capacity of the substances by integrating the Cp function and assume ideal behavior as an approximation. Use the editor to format your answer T(Kein 1000 C C, 0586 -192 ORS 10 0 -1906 -1.27 054 -019 -02 0 -0009 0.39 TABLE AG Constant-Pressure Specific Heats of various Ideal Gases! Co=C+0,0 +0,02 +Cje (kg) Gas Formula Steam C. C H2O Acetylene 1.79 0.107 CH Air 1.03 2.91 Ammonia 1.05 -0.365 NH, 1.60 Argon 14 Ar 0.52 Butane 0 C,H 0.163 5.70 Carbon dioxide CO2 0.45 1.67 Carbon monoxide CO 1.10 -0.46 Ethane CH 0.18 5.92 Ethanol C,H,OH 0.2 -4.65 Ethylene CH 0.136 5.58 Helium He 5.193 0 Hydrogen H 13.46 4.6 Methane 1.2 3.25 Methanol CH,OH 2.21 0 Neon Ne -0.031 Nitric oxide -0.48 Nitrogen N20 CH n-Octane 0.88 02 Oxygen CH -0.096 CH, -0.454 0.29 003 063 0 3.79 -0.71 -0.89 0 -0.14 -042 0A2 0.775 -0.33 NO N2 1.0 -2.31 -1.82 -30 0 -685 0.75 0.81 0 0.325 0.96 -1.31 -3.67 0.54 -3.6 -1.25 -1.35 -0.93 -0.284 -- 2.23 -0.77 0.66 1.03 0.98 1.11 0.49 -0.053 Nitrous oxide 165 6.75 -0.0001 6.95 1.47 1.87 2.27 0.73 0.36 0.35 0.041 0 Propane R-12 R-22 R-32 R-125 R-134a Sulfur dioxide Sulfur trioxide CCI,F2 CHCIF CFH CHF CF, CF,CHF SO2 SO, 0.26 0.2 0.227 0.305 0.165 0.37 0.24 1.68 2.81 1.05 1.7 1.11 0.21 0.46 -15 Approximate forms valid from 250 K to 1200 K, *Formula limited to maximum 500 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


