Question: Please Read Full Question! Different Parameters! The liquid-phase reaction A+BC follows an elementary rate law and is carried out isothermally in a flow system. The
Please Read Full Question! Different Parameters!
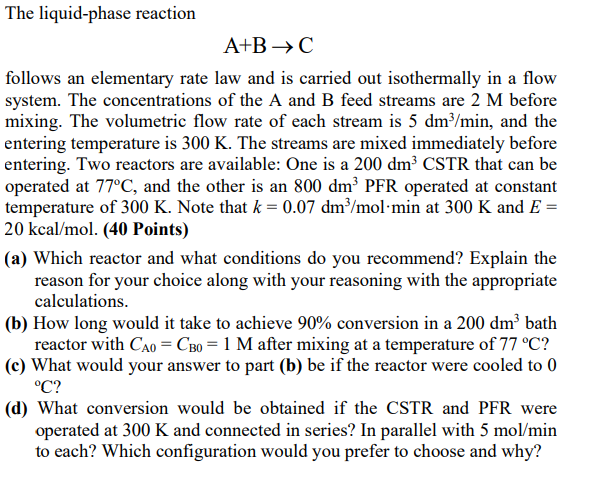
The liquid-phase reaction A+BC follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of the A and B feed streams are 2M before mixing. The volumetric flow rate of each stream is 5dm3/min, and the entering temperature is 300K. The streams are mixed immediately before entering. Two reactors are available: One is a 200dm3 CSTR that can be operated at 77C, and the other is an 800dm3 PFR operated at constant temperature of 300K. Note that k=0.07dm3/molmin at 300K and E= 20kcal/mol. (40 Points) (a) Which reactor and what conditions do you recommend? Explain the reason for your choice along with your reasoning with the appropriate calculations. (b) How long would it take to achieve 90% conversion in a 200dm3 bath reactor with CA0=CB0=1M after mixing at a temperature of 77C ? (c) What would your answer to part (b) be if the reactor were cooled to 0 C ? (d) What conversion would be obtained if the CSTR and PFR were operated at 300K and connected in series? In parallel with 5mol/min to each? Which configuration would you prefer to choose and why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


