Question: Please show a clear and complete step-by-step solution. Problem 1: There are two reactors of equal volume available for your use: one a CSTR the
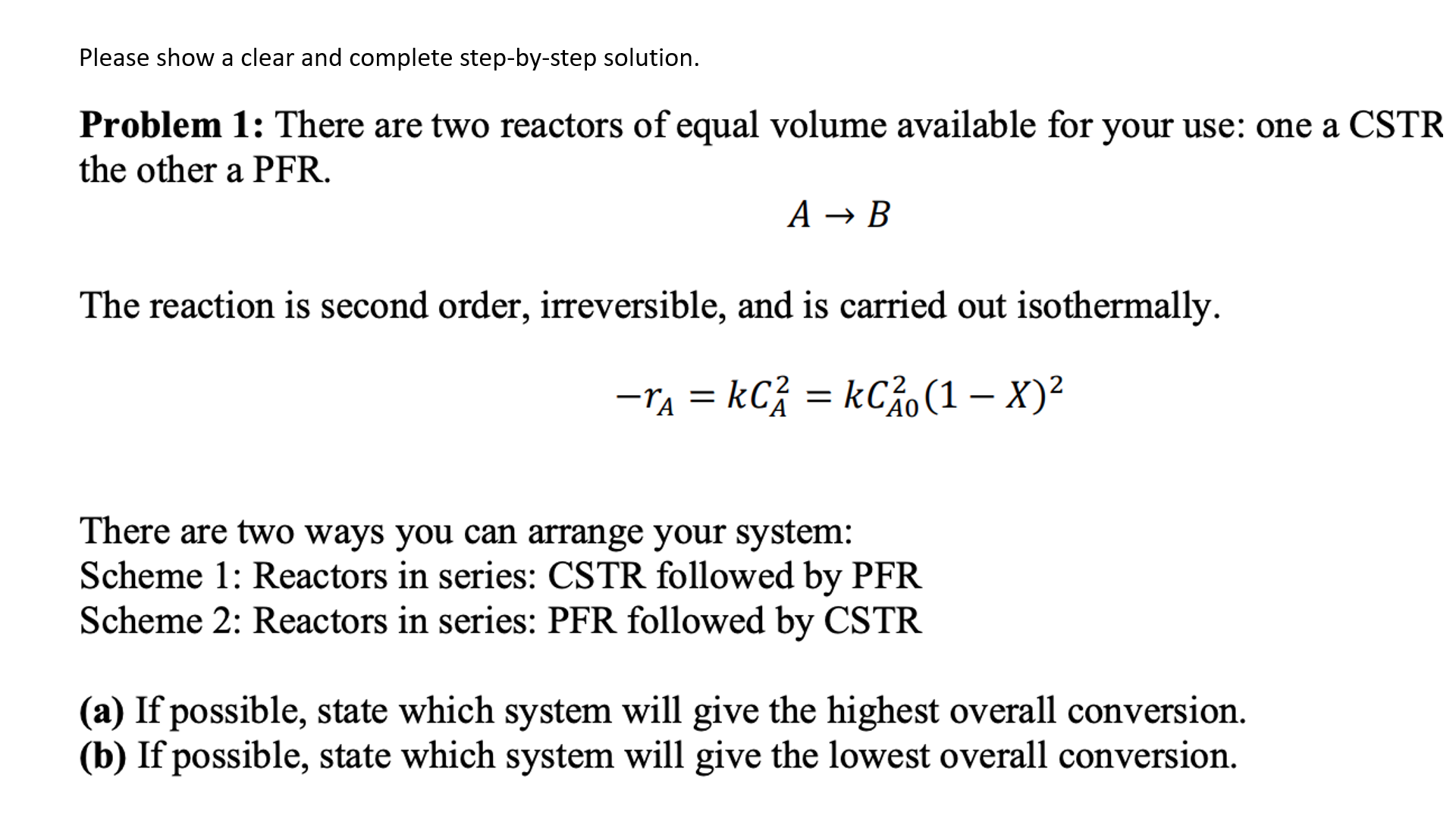
Please show a clear and complete step-by-step solution. Problem 1: There are two reactors of equal volume available for your use: one a CSTR the other a PFR. AB The reaction is second order, irreversible, and is carried out isothermally. rA=kCA2=kCA02(1X)2 There are two ways you can arrange your system: Scheme 1: Reactors in series: CSTR followed by PFR Scheme 2: Reactors in series: PFR followed by CSTR (a) If possible, state which system will give the highest overall conversion. (b) If possible, state which system will give the lowest overall conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


