Question: Please show complete step-by-step solution. This is only 1 problem. The isomerization of butane nC4H10iC4H10 was carried out adiabatically in the liquid phase. The data
Please show complete step-by-step solution.
 This is only 1 problem.
This is only 1 problem.
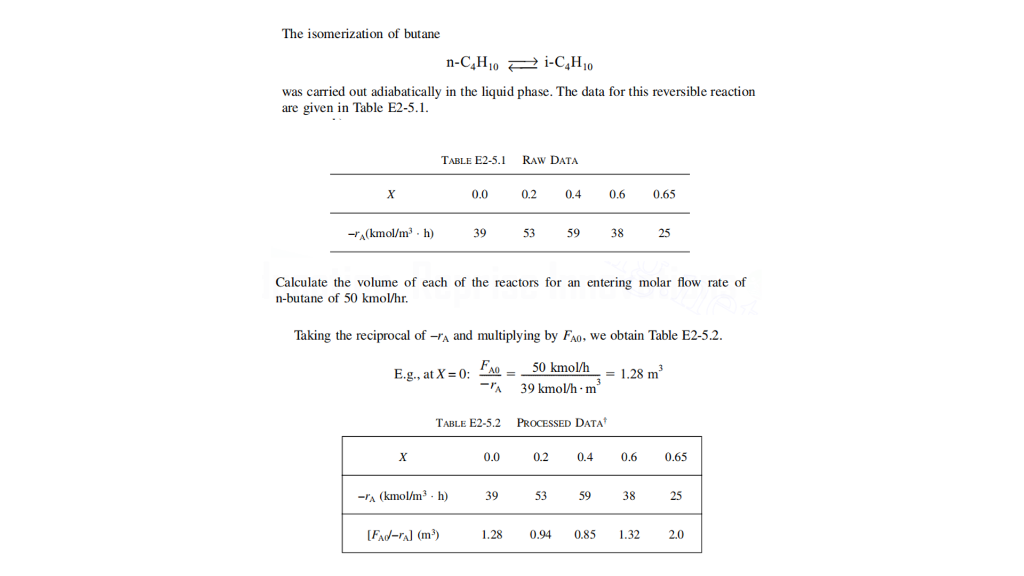
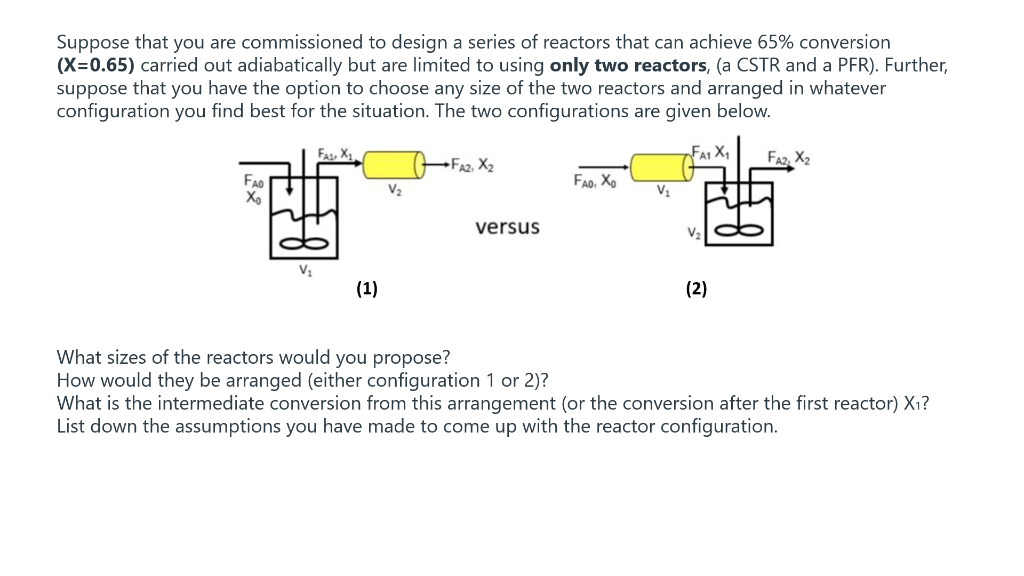
The isomerization of butane nC4H10iC4H10 was carried out adiabatically in the liquid phase. The data for this reversible reaction are given in Table E2-5.1. Calculate the volume of each of the reactors for an entering molar flow rate of n-butane of 50kmol/hr. Taking the reciprocal of rA and multiplying by FA0, we obtain Table E2-5.2. E.g.,atX=0:rAFA0=39kmol/hm350kmol/h=1.28m3 TABLE E2-5.2 Processed DATA Suppose that you are commissioned to design a series of reactors that can achieve 65% conversion ( X=0.65 ) carried out adiabatically but are limited to using only two reactors, (a CSTR and a PFR). Further, suppose that you have the option to choose any size of the two reactors and arranged in whatever configuration you find best for the situation. The two configurations are given below. What sizes of the reactors would you propose? How would they be arranged (either configuration 1 or 2 )? What is the intermediate conversion from this arrangement (or the conversion after the first reactor) X1 ? List down the assumptions you have made to come up with the reactor configuration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


