Question: Please show a detailed solution. Thank you. . EXAMPLE 11.4-1. Rectification of a Benzene-Toluene Mixture A liquid mixture of benzene-toluene is to be distilled in
Please show a detailed solution. Thank you.
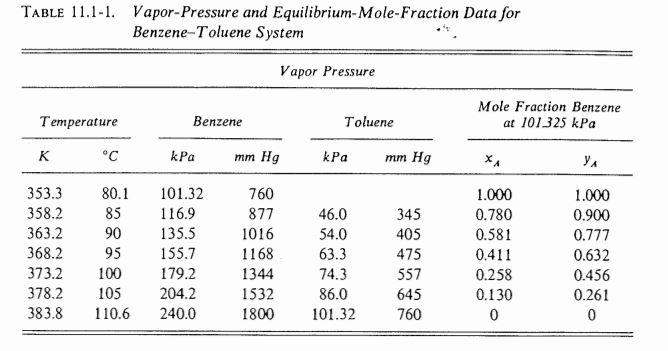
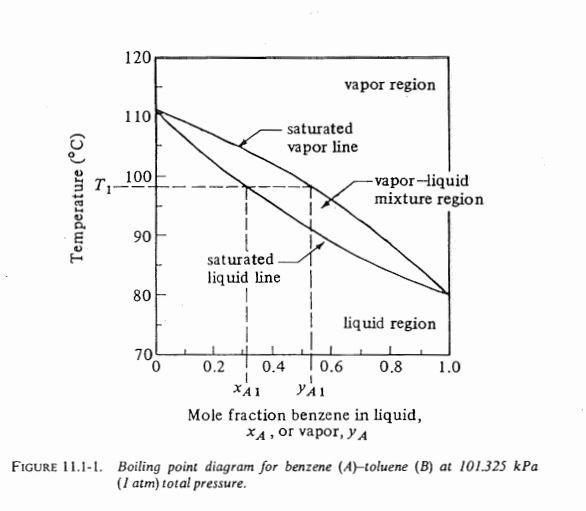
. EXAMPLE 11.4-1. Rectification of a Benzene-Toluene Mixture A liquid mixture of benzene-toluene is to be distilled in a fractionating tower at 101.3 kPa pressure. The feed of 100 kg mol/h is liquid and it contains 45 mol % benzene and 55 mol % toluene and enters at 327.6 K (130F). A distillate containing 95 mol % benzene and 5 mol % toluene and a bottoms containing 10 mol % benzene and 90 mol % toluene are to be obtained. The reflux ratio is 4:1. The average heat capacity of the feed is 159 kJ/kg mol K (38 btu/lb mol. F) and the average latent heat 32099 kJ/kg mol (13 800 btu/lb mol). Equilibrium data for this system are given in Table 11.1-1 and in Fig. 11.1-1. Calculate the kg moles per hour distillate, kg moles per hour bottoms, and the number of theoretical trays needed. Solution: The given data are F = 100 kg mol/h, xp = 0.45, xp = 0.95, 0.10, and R = L/D = 4. For the overall material balance substituting into Eq. (11.4-3) F = D + W (11.4-3) 100 = D + W = Table 11.1-1. Vapor-Pressure and Equilibrium-Mole-Fraction Data for Benzene-Toluene System Vapor Pressure Mole Fraction Benzene at 101325 kPa Temperature Benzene Toluene K C kPa mm Hg kPa mm Hg . 353.3 358.2 363.2 368.2 373.2 378.2 383.8 80.1 85 90 95 100 105 110.6 101.32 116.9 135.5 155.7 179.2 204.2 240.0 760 877 1016 1168 1344 1532 1800 46.0 54.0 63.3 74.3 86.0 101.32 345 405 475 557 645 760 1.000 0.780 0.581 0.411 0.258 0.130 0 1.000 0.900 0.777 0.632 0.456 0.261 0 120 vapor region 110 saturated vapor line 100 T Temperature (C) -vapor-liquid mixture region 90 saturated liquid line 80 1 ! liquid region 704 + 0 0.2 0.4 0.6 0.8 1.0 XA1 ya Mole fraction benzene in liquid, *A, or vapor, Y A FIGURE 11.1-1. Boiling point diagram for benzene (A)-toluene (B) at 101.325 kPa (1 atm) total pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


