Question: please show all steps A solution is prepared by dissolving 15.0g of pure HC2H3O2 and 25.0g of NaC2H3O2 in 775mL of solution (the final volume).
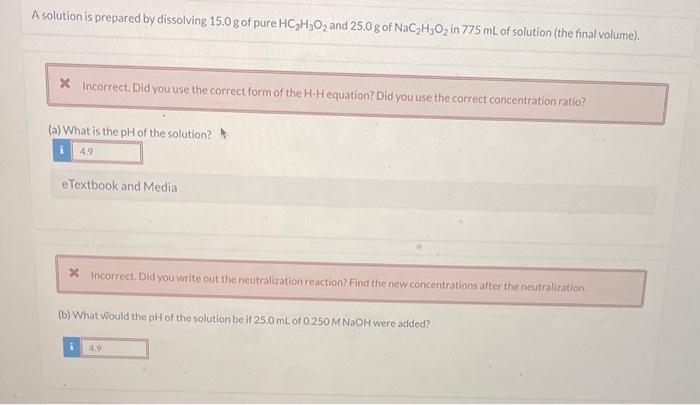
A solution is prepared by dissolving 15.0g of pure HC2H3O2 and 25.0g of NaC2H3O2 in 775mL of solution (the final volume). (a) What is the pH of the solution? eTextbook and Media x incorrect. Did you write out the neutralization reaction? Find the new concentrations after the neutralization (b) What would the pH of the solution be if 25.0mL of 0.250MNaOH were added
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


