Question: Please show all steps and explain your steps. Thank you! I promise I will like if it is correct. Problem 2 A 1 m2 tank
Please show all steps and explain your steps. Thank you! I promise I will like if it is correct.
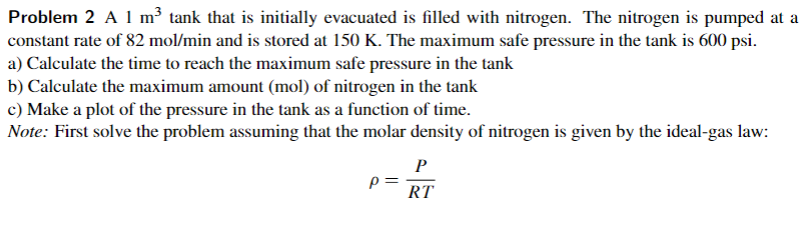
Problem 2 A 1 m2 tank that is initially evacuated is filled with nitrogen. The nitrogen is pumped at a constant rate of 82 mol/min and is stored at 150 K. The maximum safe pressure in the tank is 600 psi. a) Calculate the time to reach the maximum safe pressure in the tank b) Calculate the maximum amount (mol) of nitrogen in the tank c) Make a plot of the pressure in the tank as a function of time. Note: First solve the problem assuming that the molar density of nitrogen is given by the ideal-gas law: P P= RT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


