Question: Please show all steps and solve legibly if not typed. Thank you A silicate mineral sample is analyzed for chromium content (Cr=51.9961g/mol). The sample is
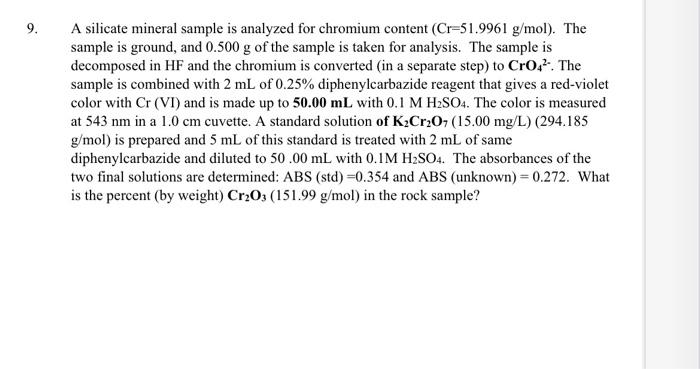
A silicate mineral sample is analyzed for chromium content (Cr=51.9961g/mol). The sample is ground, and 0.500g of the sample is taken for analysis. The sample is decomposed in HF and the chromium is converted (in a separate step) to CrO42-.The sample is combined with 2mL of 0.25% diphenylcarbazide reagent that gives a red-violet color with Cr (VI) and is made up to 50.00mL with 0.1MH2SO4. The color is measured at 543nm in a 1.0cm cuvette. A standard solution of K2Cr2O7(15.00mg/L)(294.185 g/mol ) is prepared and 5mL of this standard is treated with 2mL of same diphenylcarbazide and diluted to 50.00mL with 0.1MH2SO4. The absorbances of the two final solutions are determined: ABS(std)=0.354 and ABS (unknown) =0.272. What is the percent (by weight) Cr2O3(151.99g/mol) in the rock sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


