Question: Please show all steps for intermediates and steady-state approximations. (20 pts) One of the major reasons for engine oil degradation is the oxidation of motor
Please show all steps for intermediates and steady-state approximations.
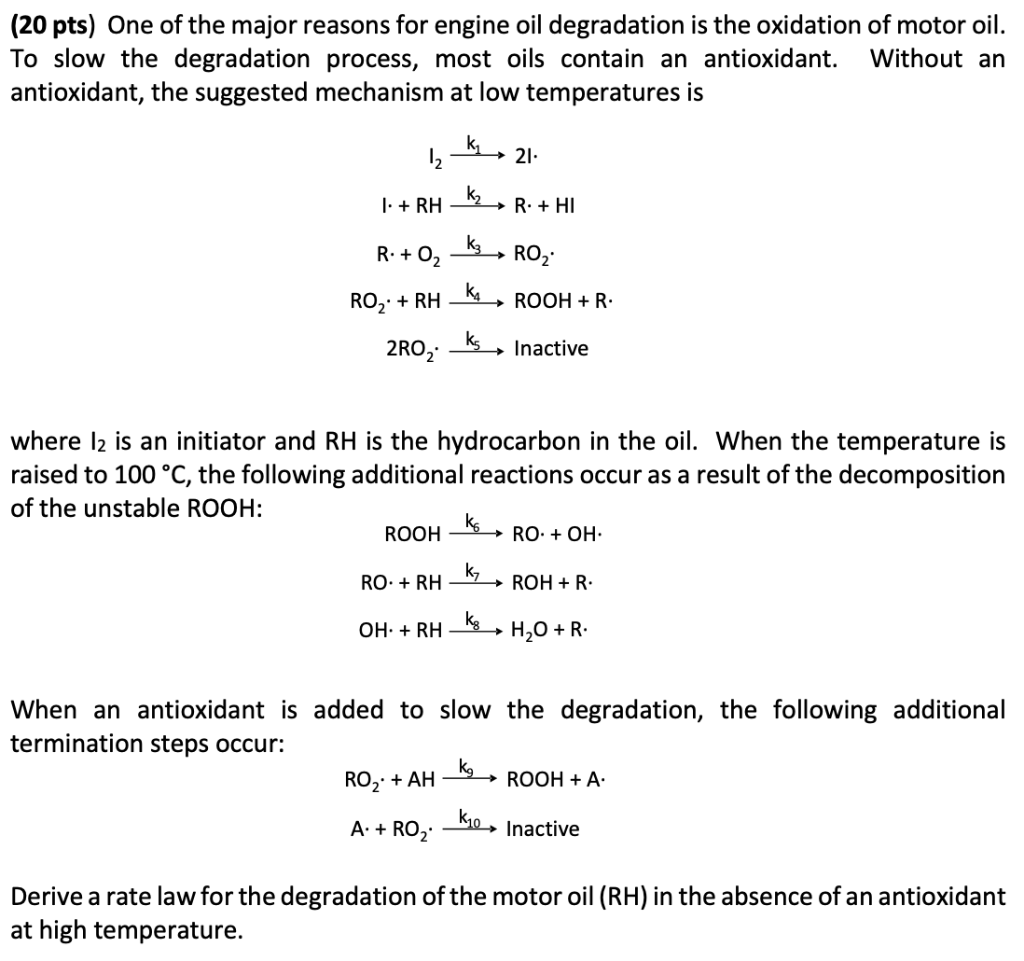
(20 pts) One of the major reasons for engine oil degradation is the oxidation of motor oil. To slow the degradation process, most oils contain an antioxidant. Without an antioxidant, the suggested mechanism at low temperatures is I2k12I.I+RHk2R+HIR+O2k3RO2RO2RHk4ROOH+R.2RO2k5Inactive where I2 is an initiator and RH is the hydrocarbon in the oil. When the temperature is raised to 100C, the following additional reactions occur as a result of the decomposition of the unstable ROOH : ROOHk6RO+OH.RO+RHk7ROH+R.OH+RHk8H2O+R. When an antioxidant is added to slow the degradation, the following additional termination steps occur: RO2+AHk9ROOH+A.A+RO2k10Inactive Derive a rate law for the degradation of the motor oil (RH) in the absence of an antioxidant at high temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


