Question: please show all the steps and don't use any math programing. if you waste my time, you simply getting a dislike and report to chegg
please show all the steps and don't use any math programing. if you waste my time, you simply getting a dislike and report to chegg
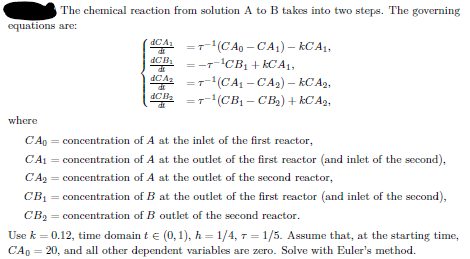
The chemical reaction from solution A to B takes into two steps. The governing equations are: CA =T-(CA-CA) - KCA1, dCB =-=-CB+CAL dC Az =--(CA1 - CA2) kC A2, dCB ==--(CB1-CB) + kC A2, where C Ap = concentration of A at the inlet of the first reactor, CA1 = concentration of A at the outlet of the first reactor and inlet of the second), CA2 = concentration of A at the outlet of the second reactor, CB1 = concentration of B at the outlet of the first reactor (and inlet of the second), CB2 = concentration of B outlet of the second reactor. Use k =0.12, time domain t (0,1), h=1/4, T=1/5. Assume that, at the starting time, CA0 = 20, and all other dependent variables are zero. Solve with Euler's method
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


