Question: Please show all work and explain it. Answer: -132+4.17x10 3 J Thank you 2.2 Most widely used empirical equations-of-state for real gas Example: One mole
Please show all work and explain it.
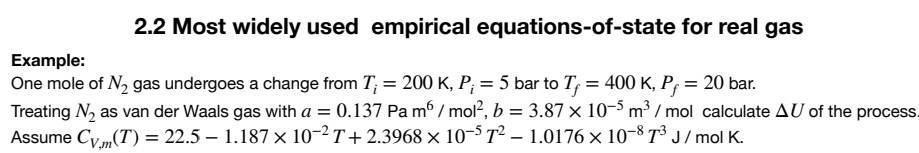
Answer: -132+4.17x103J Thank you
2.2 Most widely used empirical equations-of-state for real gas Example: One mole of N2 gas undergoes a change from Ti=200K,Pi=5 bar to Tf=400K,Pf=20 bar. Treating N2 as van der Waals gas with a=0.137Pam6/mol2,b=3.87105m3/mol calculate U of the process Assume CV,m(T)=22.51.187102T+2.3968105T21.0176108T3J/molK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


