Question: Please show all work and thoroughly explain why each statement is true The rate law for the reaction NO2(g)+O2(g)NO(g)+O3(g) is given by rate =k[NO2][O2] If
Please show all work and thoroughly explain why each statement is true
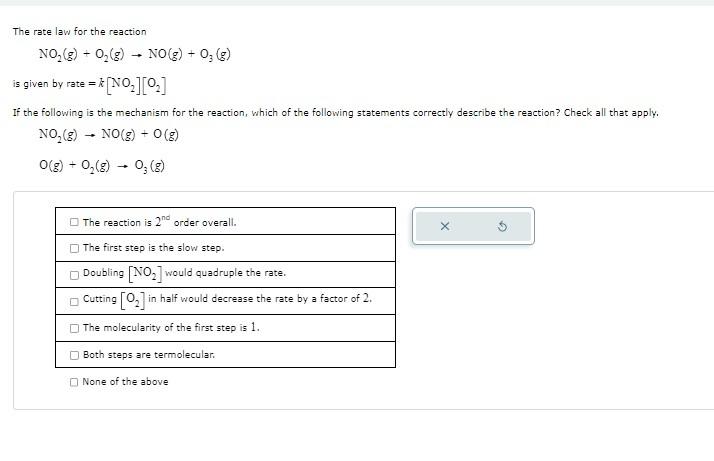
The rate law for the reaction NO2(g)+O2(g)NO(g)+O3(g) is given by rate =k[NO2][O2] If the following is the mechanism for the reaction, which of the following statements correctly describe the reaction? Check all that apply. NO2(g)NO(g)+O(g)O(g)+O2(g)O3(g) \begin{tabular}{|l|} \hline The reaction is 2nd order overall. \\ \hline The first step is the slow step. \\ \hline Doubling [NO2] would quadruple the rate. \\ \hline Cutting [O2] in half would decrease the rate by a factor of 2. \\ \hline The molecularity of the first step is 1. \\ \hline Both steps are termolecular. \\ \hline \end{tabular} None of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


