Question: Please show all work and thought process alongside. I ' m very confused and do not know where to start or what steps proceed. G
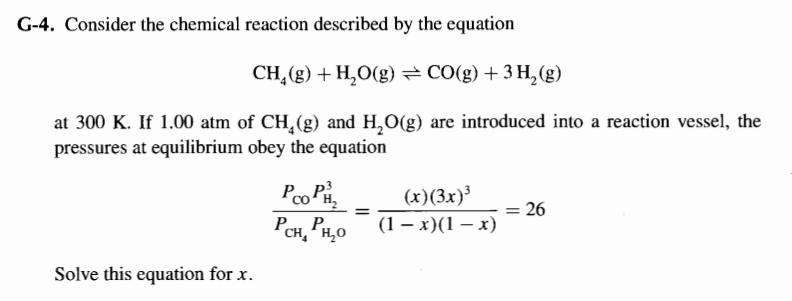
Please show all work and thought process alongside. Im very confused and do not know where to start or what steps proceed. G Consider the chemical reaction described by the equation
at If atm of and are introduced into a reaction vessel, the
pressures at equilibrium obey the equation
Solve this equation for
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


